Shruthi Suresh1, Brendan Raftery2, Bradley S. Duerstock1, 3
Weldon School of Biomedical Engineering1, Department of Computer Science2, School of Industrial Engineering3
Purdue Universityabstract
One of the more common post-SCI health conditions is Autonomic Dysreflexia (AD) and poor management of AD can be life threatening. Key physiological indicators of AD include an altered heart rate and pathological sweating above the injury site. Utilizing commercially-available galvanic skin resistance (GSR), heart rate, and skin temperature sensors, we developed a mobile computer application to enable wearers to monitor these physiological indicators in real-time and throughout the day. Subjects were recruited to regularly use the system for two weeks and report any incidences of AD. A Support Vector Machine was used to develop a machine learning model that could predict the occurrence of AD with a high level of accuracy. The objective of this study is to provide a generalizable recognition system to reliably detect the onset of AD in the vast majority of tetraplegics without prior training. Continuous physiological monitoring to alert tetraplegics and their caregivers of the onset of AD would promote greater independence and community participation.
introduction
There are approximately 17,000 new cases of spinal cord injury (SCI) in the United States alone. (National Spinal Cord Injury Statistical Center, 2016). 30-50% of persons with a SCI are rehospitalized every year due to secondary health complications. One of the most common and life-threatening post-SCI health complications is autonomic dysreflexia (AD). AD is caused by the hyperactivity of the sympathetic nervous system due to an irritation to the body below the injury site. If not managed properly, it could lead to seizures, stroke or even death. Common symptoms of AD include sweating, altered heart rate, flushing, cold and clammy skin and (Cragg & Krassioukov, 2012; Karlsson, 1999; Krassioukov, Warburton, Teasell, & Eng, 2009).
Symptoms and management vary among individuals, and tetraplegics must become familiar with their own triggers and symptoms (Linsenmeyer, Campagnolo, & Chou, 1996). In some individuals, it can be difficult to identify the cause of AD and immediate pharmaceutical medical intervention is required. However, despite the severity of AD, only 41% of persons with chronic SCI and their family had heard of it. Moreover, 22% of individuals with SCI reported symptoms consistent with unrecognized AD. (McGillivray, Hitzig, Craven, Tonack, & Krassioukov, 2009)
In previous studies we found that changes in Galvanic Skin Resistance, Heart Rate and Skin Temperature served as effective physiological indicators to identify the onset of AD (Suresh, Duerstock, & Duerstock, 2016). In this paper, we used a Support Vector Machine (SVM) based learning model to predict the onset of AD in individuals based on the aforementioned physiological parameters measured using a commercially available smartwatch. In a prior study we showed that the sensors in smartwatches were sensitive enough to reliably detect changes in the physiological parameters which indicate the onset of AD(Suresh, Shruthi; Raftery Brendan; Duerstock, 2016). However, due to variations in the way AD presents itself in individuals with SCI, thresholding is a limited method to accurately detect the onset of AD. This requires the use of a machine learning algorithm such as SVM which is a well-known data classification algorithm (Cortes & Vapnik, 1995). Using machine learning, physiological data can be collected from a handful of subjects to develop a robust telemetry system to accurately detect the onset of AD. Such a monitoring system would be beneficial to newly injured tetraplegics to better understand when AD has occurred to prevent escalation of the health condition. This system can automatically send warnings to medical personnel or caregivers through the mobile phone application to provide emergency oversight.
methods
Physiological Monitoring Wristband
A Microsoft® Band™ (MS Band) (Fig. 1A) was used to detect physiological signals such as Galvanic Skin resistance (GSR), heart rate (HR), and skin temperature (ST). The MS band was accommodated for individuals with higher level SCIs by using a modified wrist orthotic that can be worn by users for extended periods of time.
Mobile Computer Application
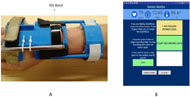
Data Collection
Physiological data was collected from three individuals with complete cervical SCIs for a duration of two weeks (Table 1).
The subjects were asked to record the data for at least 8 hours a day and self-report incidents of AD. The data collected was filtered using a moving average filter to reduce noise.
| Subject | Gender | Level of Injury | Years since injury |
| Subject 1 | M | C4/C5 | 26 |
| Subject 2 | M | C5/C6 | 4 |
| Subject 3 | F | C6/C7 | 20 |
Machine Learning Model
For this study a Python based library was used to develop a linear SVM model. SVM is a supervised machine learning classifier defined by a separating hyperplane. It uses previously labelled data points to develop an optimized hyperplane which assists in the categorization of subject data sets based on features of the data. In our system, 70% of all the data collected for each subject served as training data for the model. The remaining 30% of the data was used as test data. This test data was further randomized and split into four different test scenarios. Different combinations of the three physiological signals were used as features for the SVM to determine which physiological signals generated the most accurate machine learning model.
The outputs of the model represent the detection of AD (0 if no AD is detected, +1 if AD is detected), based on the physiological parameters. Accuracy was calculated by comparing the predicted output generated by the model to the subjects’ self-reported occurrence of AD.
The false negatives produced by the model were also calculated. A false negative was determined when the SVM model failed to detect AD in instances where the subject had reported AD.
results
Accuracy of Machine learning model
All three subjects reported AD during the two weeks. As shown in Table 2, the machine learning model detected AD with the highest accuracy (96%) when GSR and Skin temperature as the key features. The model had the lowest accuracy (93.6%) when GSR and heart rate were used. Similar accuracies were determined when the model was trained with all three physiological parameters as features and also, when only GSR was a key feature.
| Subject | GSR only | GSR+ ST | GSR+ HR | GSR+ HR+ST | |
| Subject 1 | 93.7% | 95.9% | 95.5% | 95.6% | |
| Subject 2 | 93.9% | 95.2% | 95.2% | 94.7% | |
| Subject 3 | 96.9% | 96.6% | 90.1% | 94.6% | |
| Average | 94.8% | 95.9% | 93.6% | 94.9% | |
False Negative Detection
As shown in Table 3, the lowest false negative rate (3.1%) was detected by the machine learning model which used a combination of GSR and skin temperature as key features and the highest false negatives (3.9%) resulted from the model which used a combination of GSR and heart rate.
| Subject | GSR only | GSR+ ST | GSR+ HR | GSR+ HR+ST | |
| Subject 1 | 5.1% | 3.8% | 4.4% | 4.2% | |
| Subject 2 | 2.8% | 2.3% | 3.9% | 3.5% | |
| Subject 3 | 3.1% | 3.2% | 3.3% | 3.4% | |
| Average | 3.6% | 3.1% | 3.9% | 3.7% | |
discussion
Vigilant monitoring of telltale symptoms of AD can prevent the rapid rise of AD episodes and reduce risks to personal health. However, learning to recognize AD symptoms can take time at the potential risk of the individual. Given that the most common age of sustaining a SCI is 19 years old, this population is most vulnerable to develop AD crises. New tetraplegics that may not have received adequate post-SCI management education may also be vulnerable to severe AD consequences leading to higher mortality rates.
By assisting new tetraplegics to be more aware of the potentially dangerous secondary health complications, our system could significantly reduce the incidence of re-hospitalization, decrease health care costs and potentially save a life. However, it can be difficult for tetraplegics to identify what may be occurring in their bodies due to the noxious stimuli to paralyzed parts of the body. In the case of AD, it can be particularly difficult to isolate the source of noxious stimuli, such as a full bladder or pressure sore. Through the development of this wearable telemetry system, we aim to allow individuals with SCI greater self-sufficiency and safety, which is crucial to helping persons with SCIs to successfully re-enter society and their communities.
Detecting AD with a machine learning model
AD often presents itself differently in various individuals. The most commonly known physiological indicators of AD are sweating above the injury site and cold, clammy skin. In some individuals bradycardia (slowdown in heart rate) is often observed, but tachycardia (increase in heart rate) is also common (Blackmer, 2003; Krassioukov et al., 2009; Wan & Krassioukov, 2014). In this study, we noted that an SVM based machine learning model trained using GSR and skin temperature as key features yielded the highest accuracy and the lowest false negative rate in detecting AD. However, the combination of GSR and heart rate as key features yielded the lowest accuracy in detection of AD. Due to the differences of change in heart rate upon the onset of AD in different individuals, it is preferable to train the machine learning model using a combination of GSR and skin temperature data as opposed to training with a combination of GSR and heart rate data.
The occurrence of false negatives is possibly a result of subjects forgetting to report instances of AD when they occurred. Some of the subjects in our study also reported that their threshold for detecting AD symptoms had changed over the years since their injury causing them to no longer associate certain mild symptoms with AD even though their physiological parameters respond to the external stimuli. The occurrence of false negatives detected by the telemetry system can also be attributed to this acclimatization. In future work, we can explore the development of an adaptive machine learning algorithm which can learn new thresholds based on the years since the injury.
The high accuracy of detection of AD, coupled with the low false negatives of the machine learning model allows the development of a more customized system which would assist individuals with SCIs in detecting AD and possibly notify their caregivers of an emergency.
conclusionS
The goal of this project is to develop a user-independent recognition system to detect AD in the vast majority of tetraplegics without needing to train the system. We believe that by recruiting a large number of tetraplegics to provide physiological data sets, we can develop a very robust machine learning model to detect the onset of AD in the vast majority of tetraplegics under any conditions.
References
Blackmer, J. (2003). Rehabilitation medicine: 1. Autonomic dysreflexia. Canadian Medical Association Journal, 169(9), 931–5.
Cortes, C., & Vapnik, V. (1995). Support-Vector Networks. Machine Learning, 20(3), 273–297.
Cragg, J., & Krassioukov, A. (2012). Autonomic dysreflexia. Canadian Medical Association Journal, 184(1), 66. https://doi.org/10.1503/cmaj.110859
Karlsson, a K. (1999). Autonomic dysreflexia. Spinal Cord, 37(6), 383–91. https://doi.org/10.1038/sj.sc.3100867
Krassioukov, A., Warburton, D. E., Teasell, R., & Eng, J. J. (2009). A Systematic Review of the Management of Autonomic Dysreflexia After Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation, 90(4), 682–695. https://doi.org/10.1016/j.apmr.2008.10.017
Linsenmeyer, T. A., Campagnolo, D. I., & Chou, I. H. (1996). Silent autonomic dysreflexia during voiding in men with spinal cord injuries. The Journal of Urology, 155(2), 519–22.
McGillivray, C. F., Hitzig, S. L., Craven, B. C., Tonack, M. I., & Krassioukov, A. V. (2009). Evaluating knowledge of autonomic dysreflexia among individuals with spinal cord injury and their families. The Journal of Spinal Cord Medicine, 32(1), 54–62.
National Spinal Cord Injury Statistical Center (NSCISC). (2016). Spinal cord injury facts and figures at a glance. The Journal of Spinal Cord Medicine, (February). https://doi.org/10.1179/1079026814Z.000000000322
Suresh, Shruthi; Raftery Brendan; Duerstock, B. (2016). Wearable Physiological Telemetry for Individuals with Spinal Cord Injuries to SELF-MONITOR Secondary Health Complications. In RESNA/NCART 2016.
Suresh, S., Duerstock, H., & Duerstock, B. (2016). Skin resistance as a physiological indicator for quadriplegics with spinal cord injuries during activities of daily living. In Lecture Notes in Computer Science (Vol. 9545, pp. 157–168). Springer, Cham.
Wan, D., & Krassioukov, A. V. (2014). Life-threatening outcomes associated with autonomic dysreflexia: A clinical review. The Journal of Spinal Cord Medicine.
Acknowledgements
The work presented was supported by the Indiana Spinal Cord and Brain Injury Research Fund through the Indiana State Department of Health. The authors would also like to thank Arjmand Samuel and Microsoft Research for their assistance and Regenstrief Center for Healthcare Engineering.