1 Temple University, Philadelphia; 2Northeastern University, Boston; 3Magee Rehabilitation Hospital, Philadelphia;
4MossRehab, Einstein Healthcare Network, Philadelphia
INTRODUCTION
Lack of regular physical activity (PA) is a major concern among the approximately 300,000 individuals with spinal cord injury (SCI) who are at an elevated risk of mortality due to cardiovascular diseases, diabetes, and lung disease [1]. Low levels of PA and high levels of sedentary behavior (SB) in individuals with SCI have also been associated with secondary conditions such as pain, fatigue, weight gain, and deconditioning [1, 2]. Regular PA and exercise interventions have been linked with improved outcomes and healthier lifestyles among those with SCI [2]. To assess regular PA and exercise interventions, researchers have evaluated sensor-based activity monitors that quantify movement of the individual, wheelchair movement, and physiological changes [3-5] .
Garcia-Masso et al. found that the energy expenditure estimated by the activity counts from an activity monitor (Actigraph GT3X) worn on the wrist was highly correlated with the criterion energy expenditure during housework activities, arm-ergometry, and propulsion (correlation: r=0.86) [3]. Furthermore, Kiuchi et al. found that the EE estimated by acceleration and angular velocity from an upper-arm sensor (left upper arm - variance: R2=0.75, right upper arm R2=0.87) was similar to a wrist sensor (left wrist: R2=0.86, right wrist: R2=0.68) during wheelchair propulsion on a treadmill [4]. Hiremath et al. developed a physical activity monitoring system that used a wheel rotation sensor and a wrist worn accelerometer to estimate energy expenditure for manual wheelchair users with SCI [5]. Results indicated that the system estimated energy expenditure with an error of less than 10% for various wheelchair-based PAs. Availability of a validated activity monitor system for individuals with SCI in the community is important for supporting research with this population, and could lead to commercial devices that assist individuals with SCI achieve a healthy and active lifestyle.

SB well enough to provide feedback on activity level to individuals with SCI. We hypothesized that near-real-time feedback on PA level will lead to higher PA levels in individuals with SCI.
METHODS
Participants
Five participants with SCI took part in this pilot study (goal: N=20). Participants are included if they are between 18-65 years of age, have a diagnosis of SCI, are at least 6 months post-injury, use a manual wheelchair as their primary means of mobility, selfpropel their wheelchair, are medically stable, have no active pelvic or thigh wounds (pressure injury staging/ulcers), are not pregnant, and have experience using a smartphone. All participants provide a written informed consent, approved by our University's Institutional Review Board, prior to participating in the study.
Instrumentation
PAIS uses off-the-shelf components such as a Bluetooth-based wheel rotation monitor and a wrist worn smartwatch to detect wheelchair-based PAs and SB in individuals with SCI (Figure 1 ). The wheel rotation monitor and smartwatch stream data to an Android-based smartphone. PAIS also collects self-reports via ecological momentary assessments (6 times per day) about the type of PAs an individual is performing during the day.
Participants wear an LG Urbane smartwatch on their preferred wrist, which records accelerometer data at 60 samples per second (60 Hz). Statistical characteristics, known as features, are computed for every minute from the raw data in real-time and sent to a smartphone application. The raw accelerometer data are sent to the smartphone via Bluetooth on an hourly basis. The Panobike speed sensor, a wheel rotation monitor, is secured to the wheelchair using cable ties to record wheel rotations. Each wheel rotation is captured by the sensor and transmitted to the smartphone application in real-time via Bluetooth Low Energy protocol.
A smartphone application was developed and implemented for an Android-based smartphone. Google Play services provides communication between the smartphone and smartwatch. The PAIS smartphone application uses decision trees, a machine learning algorithm, to use sensor data for classifying wheelchair-based PAs and estimating PA levels every minute (near real-time) [5]. Wheelchair-based PAs include resting, arm-ergometry, household activities, activities that may involve some wheelchair movement, wheelchair propulsion, caretaker pushing, and wheelchair basketball. The energy expenditure (EE) in kilocalories and distance travelled in miles are calculated based on near-real-time activity classification and wheel rotation readings, respectively. All sensor data and logs are encrypted, saved on the smartphone and uploaded to Google's Firebase Cloud storage on an hourly basis. Data can then be downloaded to a desktop computer and decrypted and viewed using custom desktop data visualization software. Research staff plot and assess each participant's data, checking for existence of raw data and compliance on ecological momentary assessments (EMAs). Also available to the research team are data on smartphone and smartwatch memory, battery, and phone usage, which is helpful to interpret missing data and estimate compliance. A research assistant contacts participants if there are issues related to data collection or abnormal patterns of human or wheelchair movement observed from sensor data.
Experimental Protocol
The study is being conducted in two phases, each with a duration of one month. The first phase of the study focusses on collecting baseline PA level and SB data. The second phase of the study focusses on providing nearreal-time feedback on PA level.
First Phase - Baseline data collection
Participants are provided with a smartphone, a smartwatch, and a wheel rotation monitor sensor. Participants are asked to wear the smartwatch every day for the entire day, while keeping the smartphone in close proximity of the smartwatch. They are asked to charge the smartphone and smartwatch every night. Once sensor set up is complete, an investigator helps the participant install the app, which can be downloaded from the Google Play Store onto the smartphone and smartwatch. Weight, height, age, gender and injury level (completeness and paraplegia vs. tetraplegia) are entered into the app. The participants are informed that they will receive six prompts to answer questions on the phone (i.e., the EMAs), scheduled randomly from 9am to 8pm. The EMA questions collect data about the current PA (e.g. arm-ergometry, resting). These EMAs allow us to compare the PA a participant is performing with the PA recognized by the smartphone application. Participants are instructed to continue normally with their daily routine for one month.
Second Phase - PA Level Feedback
At the start of the second month, an investigator visits the participant's home to collect survey information using the same instruments used at
baseline. Post-survey completion, a PA recommendation based on PA guidelines for individuals with SCI is provided to the participants [2]. Participants are told to do at least 20 minutes of moderate to vigorous intensity aerobic activity, twice per week. They are also encouraged to
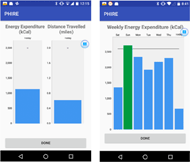
do strength training exercises, twice per week, consisting of three sets of eight to ten repetitions of exercise for each major muscle group. The clinical recommendation is intended to help the participants to improve their PA levels, health and quality of life. Participants continue to use the same sensing equipment as in month one. The smartphone app is updated to provide near-real-time feedback about EE and distance traveled for the day and the week (Figure 2). Participants proactively view the feedback.
RESULTS
Participants
Five participants have completed the pilot study to date (out of 20 total participants expected). Participants were four males and one female with a mean (SD) age of 51.6 (10.3) years, weight of 86.6 (9.8) kg and height of 1.8 (0.1) m. The injury level varied from cervical level 5 (CS) to thoracic level 10 (T10) with three of the participants having a complete injury. Table 1 shows the leisure time PA and secondary conditions.
Preliminary Data Analysis
Descriptive statistics including mean and standard deviation were analyzed for demographics, PA levels, SB, and pain. Statistical comparisons were not performed
due to the small sample size of five participants for this ongoing research study.
All statistical analyses were performed using the SPSS software (ver. 24, IBM Corp., NY).
PA Levels
| Participant | L TPAQ-SCI (Mins/davl | Pain (WUSPll | Fatiaue (FSSl | ||||
|---|---|---|---|---|---|---|---|
| Light | Moderate | Vigorous | Mean | SD | Mean | SD | |
| 1 | 14.1 | 11.6 | 0.0 | 2.9 | 0.6 | 9.9 | 2.0 |
| 2 | 68.3 | 5.8 | 16.6 | 40.9 | 14.0 | 31.3 | 4.9 |
| 3 | 52.7 | 29.1 | 22.3 | 25.9 | 8.1 | 20.4 | 8.5 |
| 4 | 43.8 | 18.6 | 0.0 | 8.1 | 4.1 | 10.2 | 3.0 |
| 5 | 47.5 | 35.9 | 32.9 | 0.0 | 0.0 | 9.0 | 0.0 |
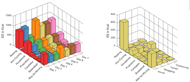
Figure 3 shows PA level patterns estimated by PAIS for various PAs for an individual with SCI. Individuals used PA level feedback, in terms of EE and distance travelled, to improve their overall PA level in the second phase of the study. Table 2 shows the PA levels and SB for individuals with SCI in the community. Participant 2 withdrew from the study, as he was unable
to use the equipment on a regular basis due to personal reasons. PA levels and SB for feedback (EE = 1792.6 (354.3) kcal, low PA= 1149.9 (245.7) minutes (mins), SB= 897.5 (256.7) mins) was higher than baseline (EE= 1692.5 (404.3) kcal, low PA= 1029.2 (259.4) mins, SB= 745.9 (252.3) mins). Moderate to vigorous PA did not change for feedback (60.0 (39.2) mins) compared to the baseline (60.3 (36.9) mins).
| Participant | EE | Total Duration | SB | Low PA | Moderate to | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (kcal) | (Minutes (Mins)) | (Mins) | (Mins) | vigorous PA (Mins) | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| 1 | 1680.5 | 359.0 | 1035.8 | 239.0 | 659.4 | 216.8 | 999.6 | 237.3 | 36.2 | 15.8 | ||
| 2 | 1344.3 | 435.3 | 1013.2 | 280.4 | 897.6 | 270.3 | 988.9 | 274.7 | 24.4 | 33.3 | ||
| Baseline | 3 | 1739.2 | 419.4 | 1090.2 | 287.1 | 848.9 | 281.6 | 1041.6 | 286.4 | 48.6 | 24.6 | |
| 1st month | 4 | 1732.9 | 432.9 | 1161.8 | 257.7 | 751.2 | 225.4 | 1069.2 | 239.6 | 92.7 | 36.3 | |
| 5 | 1564.3 | 455.6 | 1056.1 | 338.8 | 805.7 | 311.2 | 994.1 | 337.3 | 62.0 | 38.7 | ||
| 1 | 1718.4 | 386.0 | 1129.4 | 263.1 | 822.8 | 270.8 | 1101.3 | 262.3 | 28.1 | 16.5 | ||
| 2 | Lost to follow-up | |||||||||||
| Feedback | 3 | 1729.2 | 450.9 | 1105.8 | 304.8 | 892.6 | 291.1 | 1058.6 | 298.5 | 47.2 | 30.6 | |
| 2nd month | 4 | 1730.6 | 358.2 | 1168.9 | 241.1 | 754.1 | 196.5 | 1075.1 | 230.3 | 93.8 | 34.8 | |
| 5 | 1915.3 | 214.9 | 1372.0 | 102.3 | 1094.6 | 109.8 | 1307.9 | 114.0 | 64.1 | 36.1 | ||
DISCUSSION
Self-reported leisure time PA indicated that two of the five pilot study participants so far (40%) met the PA level recommendation for individuals with disabilities (150 mins/week of moderate to vigorous PA). The pain ranged from O to 41 (possible range: 0 to 150) indicating some to no interference of shoulder pain in performing regular activities. Pain reduced (N=2), maintained (N=2), or slightly increased (N=1) in the participants of the study. Fatigue severity ranged from 9 to 32 (possible range: 9 to 63), indicating no fatigue to some fatigue in the participants. Regular PA reduced (N=3) and maintained (N=2) fatigue severity in the participants of the study. Results of this pilot study are so far consistent with prior research, which linked regular PA and exercise interventions with reduced pain and improved health outcomes [2].
PA levels were higher (EE and low PA) or similar (moderate to vigorous PA) during the feedback month (Month 2) than the baseline month for this preliminary descriptive analysis. Self-awareness of PA levels has the potential to increase individuals' PA levels [9]. However, PA information alone may not motivate individuals to substantially increase their regular PA levels [9]. Providing personalized messages at specific times and contexts when an activity is being performed and proactively congratulating the participants when they achieve bouts of PAs or their goals may enhance system engagement and thereby lead to behavior change. With a system like PAIS, some factors that can be considered for personalized feedback include: i) each individual's baseline PA levels and SB, ii) PA level recommendations for that individual, and iii) the context of the PA being performed.
SB, in terms of duration (minutes), was higher during the feedback month as compared to baseline. An increase in SB is negatively correlated to health outcomes [2]. Participants' engagement with PAIS grew in the second month of the study as observed by total duration of time using PAIS (Table 2), which provided the participants with continuous but passive feedback. Further research is necessary to assess the effects of increased PA levels or bouts of PAs that disrupt long SB periods.
This paper describes the PAIS system and an ongoing pilot study. A small sample size of five participants is too small to draw conclusions, but trends are promising. We are in the process of running up to 20 participants through this protocol. Furthermore, we plan to evaluate PAIS' for just-in-time persuasive and adaptive feedback, assessing the ability of real-time feedback to motivate individuals with SCI to engage in more PA and less SB.
Our pilot has highlighted some of the challenges of conducting work with multiple, real-time sensors. Data have been lost temporarily, for example, because of participant's: i) accidentally placing their smartphones in Airplane Mode or switching off the Bluetooth, which causes data loss, and ii) connecting the smartphone to multiple Bluetooth devices such as audio systems or hands-free car phones, which disrupts the connection between the Panobike sensor and the smartphone. Due to challenges such as these, and others, the investigators have found the ability to remotely view incoming data (using the data visualization tools) to be invaluable for quickly spotting missing data or poor compliance; participants can then be contacted and the problem resolved with minimal longterm data loss.
CONCLUSION
To our knowledge, this is the first study to test a wearable computer system that can monitor PA levels and SB, and provide near-real-time PA level feedback to individuals with SCI. Our ongoing study will provide both qualitative and quantitative data to inform the development of such systems. PAIS has the potential to deliver tailored, timely, and context-sensitive feedback to people with SCI, with the goal of improving PA levels.
REFERENCES
[1) Williams TL, Smith B, Papathomas A. The barriers, benefits and facilitators of leisure time physical activity among people with spinal cord injury: a meta-synthesis of qualitative findings. Health Psychology Review, 2014 8(4): p. 404-425.
[2) Ginis KM, Hicks A, Latimer A, Warburton D, Bourne C, Ditor D, Goodwin D, Hayes K, McCartney N, Mcllraith A. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord, 2011 49(11 ): p. 1088.
[3) Garcia-Masso X, Serra-Ano P, Garcia-Raffi L, Sanchez-Perez E, Lopez-Pascual J, Gonzalez L-M. Validation of the use of Actigraph GT3X accelerometers to estimate energy expelnditure in full time manual wheelchair users with spinal cord injury. Spinal Cord, 2013 51(12): p. 898.
[4) Kiuchi K, lnayama T, Muraoka Y, Ikemoto S, Uemura 0, Mizuno K. Preliminary study for the assessment of physical activity using a triaxial accelerometer with a gyro sensor on the upper limbs of subjects with paraplegia driving a wheelchair on a treadmill. Spinal Cord, 2014 52(7): p. 556.
[5) Hiremath SV, lntille SS, Kelleher A, Cooper RA, Ding D. Estimation of energy expenditure for wheelchair users using a physical activity monitoring system. Archives of Physical Medicine and Rehabilitation, 2016 97(7): p. 1146-1153. e1.
[6) Ginis KAM, Phang SH, Latimer AE, Arbour-Nicitopoulos KP. Reliability and validity tests of the leisure time physical activity questionnaire for people with spinal cord injury. Archives of Physical Medicine and Rehabilitation, 2012 93(4): p. 677-682.
[7) Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology, 1989 46(10): p. 1121-1123.
[8) Curtis K, Roach K, Applegate EB, Amar T, Benbow C, Genecco T, Gualano J. Development of the wheelchair user's shoulder pain index (WUSPI). Spinal Cord, 1995 33(5): p. 290-293.
[9) Spring B, Pellegrini CA, Pfammatter A, Duncan JM, Pictor A, McFadden H, Siddique J, Hedeker D. Effects of an abbreviated obesityintervention supported by mobile technology: The ENGAGED randomized clinical trial. Obesity, 2017 25(7): p. 1191-1198.
ACKNOWLEDGEMENTS
The work is supported by the Craig H. Neilsen Foundation (Project# 382252). The mobile phone sensing system was made possible, in part, through funding from the National Institutes of Health (R21 HL 108018-01).