Constraint Induced Movement Therapy Device
ABSTRACT
A novel device has been developed for constraint-induced movement therapy (CIMT) in children with hemiplegia. The device immobilizes the fingers, hand, wrist, and elbow of the child's unaffected arm, forcing use of the affected arm. The device has several advantages over most current devices, which are made of standard casting material. The device is relatively inexpensive, comfortable, attractive, and easily constructed from commercially available products.
BACKGROUND
Each year, many thousands of people experience cerebrovascular accidents resulting in hemiplegia, the weakening or paralysis of one side of the body (1). Fortunately, the brain has the remarkable ability to reorganize its neurons, passing functions from parts of the brain damaged by stroke to healthy regions, allowing people with hemiplegia to regain functioning of their affected limbs (2). However, since this neural reorganization will only occur in response to external stimuli, the patient must use the affected arm to regain function.
The goal of Constraint-Induced Movement Therapy (CIMT) is to force patients with hemiplegia to use their affected arms by restraining their healthy arms. Several studies have shown significant improvement in patients who undergo CIMT (3). Most studies have involved restraining the unaffected arm using a standard cast from the upper arm to the fingertips for a few weeks in an outpatient or intensive inpatient therapy. Most therapists fabricate their own custom casts for the therapy; no standard device exists for restraint of the unaffected arm.
Problem Statement
Currently, therapists at Lenox Baker Children's Hospital (Durham, NC) use a Delta-Cast that patients undergoing CIMT wear for 3 weeks, 24 hours a day, in an outpatient intensive treatment. The Delta-Cast is a polyurethane resin cast with Velcro straps that is formed around the patient's elbow, forearm, and hand. This device has two major shortcomings. First, the Delta-Casts already in use do not cover the fingertips. Since the fingers remain exposed and the unaffected arm was not supervised, patients succeeded in deriving functionality from their casted arm. Thus, our CIMT device must offer the support of the Delta-Cast with additional restraints to prevent all use of the unaffected arm removal by the patient. The second shortcoming of the Delta-Cast is that it must be custom made for each patient. Therapists must be trained to make casts, and the fabrication process is tedious and time-consuming. The high cost of casting materials and the time involved in manufacturing make the cast expensive. Therefore, our CIMT device must be easily adaptable to multiple patients and must be more cost-effective than the current cast. The device must also be professional in appearance for parents, and both aesthetically pleasing and comfortable for the child to ensure cooperation in therapy participation.
Rationale
Providing Lenox Baker with a device that is both adaptable to multiple patients and easily reproducible will ensure the ability to accommodate a full range of patient sizes. Such a design will also be both time and cost efficient. Selecting existing commercial products for the main frame of the device will allow for easy reproduction of the device, and a professional appearance that will please parents. Using tried and proven products will ensure a comfortable fit for each patient.
Design and Development
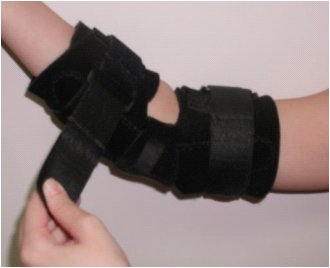 |
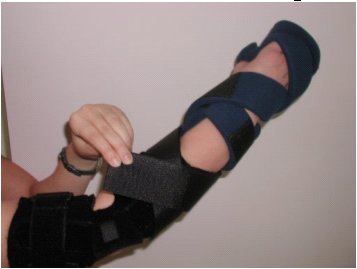
Patient and parent acceptance was a high priority for the device. Our final design therefore comprised three components to maximize ease of use for parents and to offer a sleeker appearance. The three final components of the CIMT device are: (1) elbow brace with elbow flexion/extension restraint, commercially purchased and modified with a small aluminum stay to fix its angle at 135° (Fig. 1); (2) commercially purchased hand splint made of a malleable aluminum frame with a removable lining, and added Velcro supination/pronation straps (Fig. 2); and (3) Stretchable sleeves to enclose the entire device (Fig. 3). Each component is durable and easy to put on; straps used with the hand splint are color coded to ensure correct strap direction.
 |
 |
Evaluation
Throughout the design process, author Wang wore and evaluated the comfort and appearance of the fabricated devices. Potential problems were identified and solutions were discussed and implemented. One concern that arose from these experiments was the temperature of the device in use. Thus, we performed temperature measurements in the device using three different sleeve materials. Based upon the results, we provided a lighter, more breathable sleeve alternative made out of CoolMax rather than a tight weave of Lycra and cotton.
Since minimizing the cost to the patient and therapist time were main goals for the creation of this device, we evaluated the cost and time needed to use the device throughout the design process. The final design meets these objectives and increases return on investment with each new CIMT patient as shown in table to the right.
Discussion and Conclusions
The main advantage of our CIMT device is that it meets its functional objectives while being cost and time efficient, easily reproducible, adaptable to a wide range of potential patients, and easy for parents and patients to use. Therapists do not need to be specially trained to use this device, and customizations made to the device will occur during patient fitting and patient/parent education, and will not involve additional construction. The device conforms to the needs of the therapy. The sleeve can be removed easily by the therapist or parent to monitor the skin, but can be difficult for the child to remove by knotting the drawstring enclosure if the child becomes tempted to remove the device. The only foreseeable disadvantage is if the chosen commercial products become unavailable. However, similar commercial products could be easily substituted into the device. The final device meets all objectives, and will provide a re-useable and cost-effective therapy tool to help patients with hemiplegia to regain motor skills.
We demonstrated the device for our professor, classmates, supervisors, and others familiar with occupational therapy and rehabilitation engineering on several occasions. We discussed the changes they suggested and altered our device when necessary. While the device has not been used in therapy because an appropriate client has not yet been identified, we believe that any problems discovered in clinical use of the device will be easy to correct due to the adaptability of the device.
Acknowledgements
We would like to thank Dr. Larry Bohs, our professor, for providing us with this design opportunity and aiding us in the design process. Mark Palmeri, our Teaching Assistant, aided in providing feedback and sourcing information. We would also like to thank our supervisors, Jodi Petry, Erin Eadry, and Dr. Gordon Worley, who advised us on device characteristics.
Kyle
Smith
14510 Lightning Ridge Run
Fort Wayne, IN 46814
Lynn Wang
1341 Cabrillo Ave.
Burlingame, CA 94010
