ABSTRACT:
Intrinsic hand muscles are important for dexterity and precision movements; their deterioration leads to a profound functional loss of the hand. The manual muscle test, the traditional method to assess intrinsic hand muscle strength, is subjective and lacks sensitivity. Previous devices report high interobserver error (52%) and extensive clinician involvement. We have developed a novel prototype capable of comfortably and accurately measuring IHMS for a wide range of hand morphologies and sizes. The Peg Restrained Intrinsic Muscle Evaluator (PRIME) is composed of a pegboard base, a force transducer enclosure and a display unit that utilizes a PDA loaded with a custom LabVIEW™ program. PRIME is capable of determining peak force in the range of 0-10 lbs (resolution of 0.25%) and producing a patient specific data file. Preliminary validity testing yielded a coefficient of variation for test and re-test experimentation of only 1.8% (n=5). Interobserver variation was less than 2% (n=5).
KEYWORDS:
intrinsic hand muscles, pegboard, interobserver error, muscle strength
INTRODUCTION:
Intrinsic hand muscles are of high clinical value. In the U.S., the yearly cost of treating upper extremity disorders was estimated at $18 billion (1). Each year, 1 million U.S. workers receive treatment for acute hand injuries (2). For children, the hand is the most frequently injured part of the body (3). Beyond blunt trauma, the assessment of intrinsic hand muscle strength (IHMS) is necessary for debilitating diseases like rheumatoid arthritis (4) and median/ulnar nerve injuries (5). Clinicians can use IHMS to diagnosis early signs of neurodegeneration in spinal cord injury, a leading cause of disability in the United States and other myelopathic conditions such as syringomyelia and cervical spinal cord tumors (6). Ultimately, IHMS can aid medical decision making (7), evaluate the relative benefits of surgical interventions (1, 8) and track rehabilitative progress (9,10).
The manual muscle test (MMT) is the most common clinical test to assess intrinsic hand muscle strength (11-13). They are a routine part of every evaluation of hand patients (Dr. Gloria Gogola, personal communication, March 25th 2009). Though easy to implement, the 5-point grading scale of the MMT exhibits low validity, poor reliability and inherent subjectivity (14-16). Pinch and grip devices serve as an additional proxy for IHMS (17, 18). However, these measurements are dominated by extrinsic muscles and fail to isolate the strength of individual intrinsic hand muscles (19, 20). Several hand-held devices have been designed to specifically measure IHMS. Most notable include the Intrins-o-meter (21), the Rotterdam Intrinsic Hand Myometer (8) and an apparatus developed by Pataky et al. (22). The Intrins-o-meter and the Rotterdam Intrinsic Hand Myometer require extensive clinician involvement and force patients into contorted positions (22) that lead to high interobserver error ranging from 37-52% (23). The Pataky device address some of these precision issues but requires users with technical expertise to operate, does not allow for thumb testing and precludes the testing of pediatric patients and patients with abnormal hand morphologies. In this paper, we report the development of a novel device that enables clinicians to accurately and reliably determine IHMS for adults and children.
PROBLEM STATEMENT AND PROJECT OBJECTIVE:
There is a need to accurately, reliably and conveniently quantify the IHMS of all the fingers for a wide variety of hand sizes and morphologies. Our project objective is to develop a medical device that addresses this problem statement.
METHODS:
To deliver a viable device, we outlined key objectives in terms of design criteria for our device. The design criteria focused optimizing the three main characteristics of portability, clinical utility and device sensitivity. In Table 1, PRIME’s specific design criteria are listed in detail.
Design Objective |
Design Estimate |
Target Criterion |
|---|---|---|
Weight of less than 8 kg |
Sum masses of components |
W < 8 kg |
Volume less than 0.125 m3 |
Calculate perimeter of base * height |
V < 0.125 m3 |
Durable for repeated use of up to 1 year |
Fatigue testing and accelerated use for Y time |
Y > 1 year |
Test requires < 10 minutes per hand |
Time the lag from the test start to data readout |
T < 10 min |
Interobserver error under 10% |
Collect data with different users for one patient |
5% F1<F2< 5%F1 |
Cost for prototype development < $2,000 |
Sum costs for each component |
C < $2,000 |
Clear display either in LCD or computer interface |
Various users agree is disagree about the display clarity |
> 75% agree |
Device rated from 0-8 lbs |
Use known weights to determine range |
S from 0.1-150 N |
Calibration must take less than 1 hr |
Time the lag from non-calibrated to fully calibrated mode |
Tcal < 1 hr |
Costs less than $100 per use |
Sum costs for each test including wear and tear |
Cuse < $100 |
Store data in convenient format for EXCEL analysis |
Test the compatibility of data readout with EXCEL |
Compatible w/ EXCEL |
Uses commercially available batteries, U.S. 120V AC outlet or USB plug |
Test the operation of the device with each of these power supplies |
Compatible w/ sources listed |
Less than 1 in 100,000 uses where patients suffer from physical harm |
Measure the adverse patient reaction after widespread adoption of device |
Injuries < 1 per 100,000 uses |
Table 1. The design criteria were established to create an appropriate framework for the development of the device. Our prototype has succeeded in the meeting all of the design criteria we have tested for thus far. Further validation is still required within an actual hand clinic.
Solutions considered:
Several proposals were outlined after discussions with orthopedic surgeons and hand therapists. These devices are listed in Table 2.
| Title | Description | |
|---|---|---|
A |
Bending Peg Board Strain Gauge System |
The device implements bending pegs with embedded strain gauges in order to record forces created from movements of fingers and thumb against the bendable peg. The pegs are placed in strategic located on top of the peg board. |
B |
Wrist Strapped Single Loop Load Cell System |
The device implements one loop that is connected to a load cell in order to record forces created from the movement of the finger or thumb against the loop. The wrist is strapped to the device in order to limit movements. |
C |
Peg Board Spring Sensing Optical System |
The device implements a camera in order to record the amount of displacement created when the finger or thumb pushes against the spring-loaded block. The block would be painted in a contrasting color to the rest of the device, so that its movements would be easily tracked and analyzed. |
D |
Self-Contained Strain Gauge Device |
This device included a reference rod that is attached to the forearm of the patient. This would allow the device to be “self-contained” on the patient. A loop strap would be attached to the reference rod and interface with the patient’s fingers. A built in strain gauge of the reference rod will be the force transducer unit. |
E |
Hand Muscle Imparted Fluid Turbidity Assay |
The device employs a dilitant fluid in order to measure the forces created from the movements of the finger and thumb while submerged in the fluid. The viscosity change of the fluid due to the specific finger movements can be tracked and correlated to force. |
Using a decision tree, we ranked our possible designs using our most important design criteria without weighting. This allowed us to eliminate proposal E as a possible design. We implemented a Pugh matrix to select the final design. The results showed us that a final design combining the pegboard in proposal A with the load cell of proposal B would best meet the design criteria.
Final design description of PRIME:

Figure 1. The full assemblage of PRIME is shown below in operation. A commercially available cable allows the force transducer to interact with the PDA display unit. The components of the device logically follow its operation. After the user’s individual fingers are engaged with the Velcro finger strap, the hand is restrained to the peg board. Then, the force transducer sends the voltage signal to the PDA. (Click for larger view)
The completed device is shown in Figure 1. PRIME is a self-contained, battery powered device that is capable of sensing forces up to 10 lbs or 45 Newtons at a resolution of ±0.025 lbs (Figure 1). The prototype is described in three components: the pegboard base, the force transducer enclosure and the display unit.
The pegboard base provides structural support while minimizing excess hand movement:
The custom PVC pegboard measures 2 feet in length, 1 foot in width and ½ inch in thickness. The holes are drilled in a staggered design one inch apart. Rounded pegs are a novel and rapidly adjustable method to restrain excess hand movement to improve accuracy, isolate individual fingers for highly specific testing and minimize clinician involvement. Locking the movement of the hand grants two key benefits. First, true isolation of individual intrinsic finger strength can be obtained. Second, the numerous placement holes for the plastic pegs allow for greater flexibility and rapidity in testing. Finally, PVC is a light, safe and easy to clean material.
The force transducer enclosure is a self-contained module of PRIME:

Figure 2. The load cell is an integrated strain gauge that includes all of the compensation, balancing and conductive elements built in. (A) The OMEGA® LCL – 010 Load Cell offers the desired force range of 0-10 lbs. On the top, the four strain gauges in a bridge configuration allows for the sensation of microstrain. (B) A CAD Rendering of the LCL-010 with the proper mounts, allowing for the most sensitive measurement. Force is directed along the arrows, causing an S-shaped deformation to occur. (C) The load cell in the proper mounting rests in the bottom portion of the plastic enclosure. (Click for larger view)
Shown in Figure 2, the force transducer enclosure (FTE) includes an OMEGA (Stamford, CT) LCL-010 load cell, custom circuit board and a 9 volt battery. Chosen for its high sensitivity, the OMEGA LCL-010 senses forces from 0 to 10 lbs at a resolution of 0.25%. The load cell outputs to a custom developed circuit board (Figure 3) where an AD620 instrumentation amplifier augments the voltage output of the load cell (Equation 1).
Equation 1: (G = 1 + (4.6kΩ/R2))
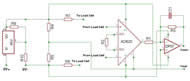
Figure 3. The circuit diagram of the amplification and filtering of our signal from the load cell. The PP3 9V battery is passed through a voltage regulator before being used to power the op amps and the load cell. The instrumentation amplifier senses the difference in voltage from the load cell caused by pulling from the patient; the load cell leads are processed through an AD260 instrumentation amplifier which gives the device a gain of 1000. The signal subsequently passes through an OP07 operational amplifier which acts as an active low-pass filter for a total gain of 2200 and fc of 28 Hz. The signal is then compared to the midrange of the voltage output by the voltage divider. (Click for larger view)
A voltage regulator and voltage divider ensure that the voltage given to the load cell and instrumentation amplifier is constant. The signal then enters an active low pass filter to eliminate any high frequency noise above 28 Hz (Equation 2).
Equation 2: ƒ = 1/2π![]() RC
RC
The voltage difference between Vout+ and Vout- is capped at 3.5 volts protecting the subsequent circuitry from damage. A D-15 output plug collects the outputs of the load cell for force measurement and the battery life of the 9 volt battery. A switch controls the power for PRIME. In addition, a LED provides visual cue of PRIME’s activation.
Interface between the FTE and pegboard base maximizes ease of use:
Figure 4. The interface between the FTE and the peg board allows for high testing flexibility. Changing the height and orientation of the FTE requires minimal clinician involvement. (Click for larger view)
The interface between FTE and the peg board base is made through a custom sleeve that allows for free rotation and height adjustment about the vertical axis (Figure 4). A helical nut allows the metallic bolt to fit tightly into the PVC board. This configuration confers several advantages. First, the vertical position of the FTE can be easily adjusted. The clamp firmly holds the FTE at a specific height. Secondly, the FTE can swivel 360° about the metallic bolt. The clinician can easily accommodate PRIME to meet various hand sizes and morphologies. Also, this connection strategy enables the measurement of intrinsic strength of the thumb which improves upon previous devices. Furthermore, this interface drives the symmetry of the peg board. By rotating the FTE, the clinician can measure intrinsic hand muscle strength in both hands without forcing the patient into new or awkward positions. To interface between the FTE and a patient’s physician, a Velcro strap is employed. The Velcro strap is a low-cost, adjustable and highly effective method to interface between a patient’s individual finger and the metallic eye-bolt connected directly to the load cell (Figure 1). In addition, the Velcro strap eliminates the risk of slippage.
Display unit utilizes National Instrument’s technology and a PDA:
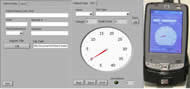
Figure 5. Tabbed browsing allows for a more efficient use of space: the first tab represents data entry for the patient; the second tab is the actual force measurements. A gauge is an intuitive way to illustrate changes in force. A low a battery indicator is shown as well. The user may select the hand and test type done. It allows the clinician to fill in data if necessary but also run the test alone. (Click for larger view)
A National Instrument’s (Austin, TX) CF-6004 CompactFlash Data Acquisition Unit was modified to sense analog voltage signals through the D-15 output directly from the FTE. The CF-6004 is placed within a HP iPAQ hx2000 PDA with a CompactFlash II slot. A custom user interface (Figure 5) developed using LabVIEW™ mobile module continuously samples voltage, automatically calculates peak force, displays the value on a digital gauge and warns of low battery.
Patient Name: Jane Doe
D/T: 03 17 09 0951PM
Operator: John Smith
Age: 02561985
Dominance: Right
Records #: 563256
Right Hypothenar Abd 1.234 kg
Left 1st DI Abd 1.453 kb
Right Intrinsic + Index 1.845 kg
Right Intrinsic + Middle 1.643 kg
Left Intrinsic + Ring 1.543 kg
Left Intrinsic + Small 1.498 kg
Right Thumb + Palmar Abd 2.434 kg
Figure 6. This sample text file is outputted by PRIME. The file name would be: 563256 (medical records number), <date> <time>.txt. This format allows the clinician to download large amounts of patient files into a database for further analysis.
Tabbed browsing allows the clinician to input critical patient data and export it in .txt format for further analysis. The underlying program architecture is composed of the two components; one loop controls voltage sampling from the load cell and battery and the second loop handles data storage and export regarding the patient’s information. A new file is generated every testing session and automatically named by the patient’s medical record number and a time stamp (Figure 6).
The advantages of the NI CF-6004 and PDA display include highly accurate sampling, ultra-portability and minimal technical expertise for operation. LabVIEW™ is a powerful programming language that is capable of generating executables that run on any mobile platform. The ability to store data in a standardized format is critical for scalability.
RESULTS:
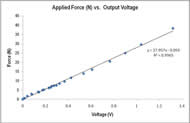
Figure 7: Output voltage from an applied force on the load cell yielded a strong linear fit. More weights were used at the lower weight spectrum to ensure sensitivity. (Click for larger view)
PRIME’s technical characteristics and testing protocol allow it to accurately and repeatedly measure intrinsic hand muscle strength. Calibration produced a linear fit with a r2 > 0.99 (Figure 7), indicating high confidence in extrapolated force measurements.
Test-retest was used to measure single observer repeatability. In this scenario, one observer measured the small finger abduction strength of a single subject five times with enough time to recover between tests. Comparing the force standard deviation to the mean yielded a coefficient of variation of 1.8% (Table 3). To determine interobserver repeatability, the small finger abduction strength of a single subject was measured by three trained observers and one untrained observer (Table 4). The coefficient of variation for all four observers was found to be 6.7%. The coefficient of variation for trained observers was only 1.4%. These values suggest that PRIME allows for highly repeatable measurements for a single observer-subject combination as well as for multiple observers testing the same subject.
Test-retest |
Force (N) |
Test 1 |
14.66 |
Test 2 |
14.96 |
Test 3 |
14.35 |
Test 4 |
14.70 |
Test 5 |
14.99 |
Average |
14.703 ± 0.26 (standard deviation) |
Interobserver |
Force (N) |
Observer 1 (trained) |
16.642 |
Observer 2 (trained) |
16.34 |
Observer 3 (trained) |
16.80 |
Observer 4 (not trained) |
17.16 |
Overall Average |
16.60 ± 0.34 (standard deviation) |

Figure 8: This market survey tested clinicians, hand therapists and nurses who would be highly likely to use PRIME. Overall, the response for PRIME was positive in each area questioned. (Click for larger view)
A survey was implemented to measure clinician interest and satisfaction with PRIME on a visual analog scale from Strongly Agree to Strongly Disagree for various statements. Overall, feedback from nurses and doctors at Shriners Hospital for Children™ in Houston was highly positive (Figure 11). This reflects PRIME’s ability to generate physician buy-in within a clinical setting.
In addition, PRIME was tested with actual pediatric patients in a small focus group. The patients were capable of following directions and comfortably completing all the tests that included the thumb.
CONCLUSIONS:
PRIME is driven by a pressing clinical need. Without question, this device holds value for patients suffering from hand injuries and various neuromuscular diseases that lead to disability. In particular, PRIME will be a valuable tool to track rehabilitation progress. The design of PRIME will continue to maximize usability and deliver high quality clinical data. Although the prototype is fully functional and ready for clinical testing, we expect to continually add to and improve upon our design.
FUTURE STEPS:
Currently, we are undergoing institutional approval from Shriners Hospital for Children™ to conduct a full validation study for publication in a peer-reviewed journal. A provisional patent has been filed and we plan on commercialization. The initial prototype cost $900 to develop (Table 5). With high volume and certain modifications, we estimate a price point of under $200 (Table 6).
PRIME Prototype |
|
Component |
Price per unit |
|---|---|
PVC pegboard base (1”x2”) |
$23.56 |
5/16” Nylon pegs |
$1.60/6 pegs |
Spring clamp |
$2.08 |
Velcro strap |
$0.09/each |
Plastic enclosure |
$5.95 |
Load transducer screw |
$0.35 |
Circuit board |
$25 |
PDA (HP Ipaq HX2400 Series Pocket PC) |
$150 |
CF-6004 DAQ card and connector cable |
$679 |
Total |
$887.63 |
PRIME: Microcontroller |
|
Component |
Price per unit |
PVC pegboard base (1”x2”) |
$23.56 |
5/16” Nylon pegs |
$1.60/6 pegs |
Spring clamp |
$2.08 |
Velcro strap |
$0.09 |
Plastic enclosure |
$5.95 |
Load transducer screw |
$0.35 |
Circuit Board |
$25 |
Microcontroller with LCD display |
$4.28 |
PearlBlue Bluetooth module |
$99 |
Total |
$161.91 |
REFERENCES
- Dias, JJ, Garcia-Elias M. (2006). Hand injury costs. Injury, 37, 1071-1077.
- Sorock GS, Lombardi DA, Courtney TK, Cotnam JP, Mittleman MA. (2001). Epidemiology of occupational acute traumatic hand injuries: a literature review. Safety Science, 38, 241-256.
- Hastings H II, Simmons BP. (1984). Hand fractures in children. A statistical analysis. Clin Orthop Relat Res, 188, 120-130.
- Estes JP, Bochenek C, Fassler P. (2000). Osteoarthritis of the fingers. J Hand Ther, 13, 108–23.
- Lundborg G. (1993). Peripheral nerve injuries: pathophysiology and strategies for treatment. J Hand Ther 1993, 6, 179–88.
- Jacquemin GL, Burns SP, Little JW. (2004). Measuring hand intrinsic muscle strength: normal values and interrater reliability. J Spinal Cord Med, 27, 460-7.
- Heras PC, Burke FD, Dias JJ, Bindra R. (2003). Outcome measurement in hand surgery: report of a consensus conference. Br J Hand Ther, 8, 70-80.
- Schreuders TA, Selles RW, Roebroeck ME, Stam HJ. (2006). Strength measurements of the intrinsic hand muscles: A Review of the Development and Evaluation of the Rotterdam Intrinsic Hand Myometer. J Hand Ther, 19, 393-402.
- Brandsma JW, Schreuders TAR, Birke JA, Piefer A, Oostendorp R. (1995). Manual muscle strength testing; intraobserver and interobserver reliabilities for the intrinsic muscles of the hand. J Hand Ther, 8, 185-190.
- Rosen B, Dahlin LB, Lundborg G. (2000). Assessment of functional outcome after nerve repair in a longitudinal cohort. Scand J Plast Reconstr Surg Hand Surg, 34, 71–8.
- Merlini L, Mazzone ES, Solari A, Morandi L. (2002). Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve, 26, 64-70.
- Taylor NF, Dodd KJ, Graham HK. (2004). Test-retest reliability of handheld dynamometric strength testing in young people with cerebral palsy. Arch Phys Med Rehabil, 85, 77-80.
- Ottenbacher KJ, Branch LG, Ray L, Gonzales VA, Peek MK, Hinman MR. (2002). The reliability of upper- and lower-extremity strength testing in a community survey of older adults. Arch Phys Med Rehabil, 83, 1423-7.
- Kaufman K, An KN, Litchy W, Morrey BF, Choa EY. (1991). Dynamic joint forces during knee isokinetic exercise. Am J Sports Med, 19, 305-16.
- Chan KM, Maffulli N, Korkia P, Li CT. (1996). Principles and practice of isokinetics in sports medicine and rehabilitation. Hong Kong: Williams & Wilkins.
- Kannus P. Isokinetic evaluation of muscular performance: implication for muscle testing and rehabilitation. (1994). Int J Sports Med, 15, S11-8.
- Richards L, Palmiter-Thomas P. (1996). Grip strength measurement: a critical review of tools, methods, and clinical utility. Crit Rev Phys Rehabil Med, 8, 87-109.
- Swanson GA, Swanson GG, GoÈ ran-Hagert C. (1995). Evaluation of impairment of hand function. In: Hunter JM, Mackin EJ, Callahan AD (Eds) Rehabilitation of the hand: surgery and therapy. St Louis, C.V. Mosby.
Shuai “Steve” Xu
BSc in Bioengineering | Rice University
2211 S. Braeswood Blvd., Unit 23B
Houston, TX 77030
Cell: 909-964-7006
Email:stevexuster@gmail.com