1 Neuroscience Cognitive Center CNRS UMR 5229, University of Lyon & Le Vinatier Hospital, France
2 Fernand Seguin Research Centre, University of Montreal & Louis-H. Lafontaine Hospital, Canada
3 DOMUS Laboratory, University of Sherbrooke, Canada
Mobus group is composed of :
Psychiatrists at Louis-H. Lafontaine Hospital (Quebec, Canada): Bentaleb, L.A., Landry, P., Lipp, O., Tranulis, C., Villeneuve, M.; Neuropsychologist at the Vinatier hospital (Lyon, France): Jacquet, P.; Ergotherapists at Louis-H. Lafontaine Hospital (Quebec, Canada): Cloutier, C., Lalancette, C., Prince, A.; Pharmacists at Louis-H. Lafontaine Hospital (Quebec, Canada): Vincent, P., Lum, M.; Social worker at Vinatier hospital (Lyon, France): Berrube, M.C.; Beneficiaries attendant at Louis-H. Lafontaine Hospital (Quebec, Canada): Lucas, M.; Nurses at Vinatier hospital (Lyon, France): Boisset, G., Guida, M., Mazuire, J., Meylan, F., Meynier, J., Pelletier, G, Sportiello, S.; Programmers at University of Sherbrooke (Québec, Canada): Giroux, S., Marcotte, N., Pigot, H., Viboud, J.P.; Students at University of Montreal (Quebec, Canada): Doré-Gauthier, V., Guévremont, C., Nadeau-Marcotte, F.
Abstract
Our aim was to investigate the use and the appreciation of Mobus, an assistive technology for cognition, by nine schizophrenia outpatients who tested the device during six weeks. Mobus provides two interconnected applications. The patient-application allows the planning of activities and reporting of symptoms. The caregiver-application provides monitoring of activity management and of the symptom reports. Patients found Mobus easy to use and validated 40% of their activities and signalled more than one symptom per week, the majority after noon. Mobus provides useful ecological data for the follow-up of the daily activities as well as the evolution of the patient’s symptoms. Connection breakdown limited the appreciation of Mobus as well as the possibility to improve neuropsychological assessment scores. This pilot study allowed us to improve the device. The enhanced version is currently being tested with a larger cohort and a control group.
Key words:
Schizophrenia; assistive technology; autonomy; executive functioning; activity of daily living
INTRODUCTION
Schizophrenia is defined as a chronic psychosis that affects approximately 1% of the world's adults. It is characterized by positive (hallucinations, delusions, disorganized behaviour…) and negative symptoms (social avoidance, blunted affect, apragmatism…). Antipsychotics help to control these symptoms, but cognitive impairments remain by most of schizophrenia patients, and have functional impacts (1, 2). In particular, deficits in memory and executive functions lead to difficulties in planning Activities of Daily Living (ADL) (3, 4). Most of patients are therefore unable to find or keep a job, establish interpersonal relationships, or live on their own. Patients also lose their autonomy, and suffer from social handicap.
The evolution of new technologies contributes to provide remote services for patients as well as for caregivers by developing interactive assistance systems. Assistive Technology for Cognition (ATC), also referred as a “cognitive orthotic” or “prosthetic” (5, 6) can be considered as a “crutch for the mind”. It has the advantage of being highly customizable, so that one can directly assist each patient in performing his ADL (6, 7). The efficacy of ATC has been proven for people suffering from mnesic and executive deficits due to neurodevelopmental and neurodegenerative illnesses, brain lesions (8, 9) and mental retardation (6, 10). To our knowledge, there are no ATC available to improve the autonomy of schizophrenia patients yet.
Objective
Our aim was to explore the use and the appreciation, by schizophrenia patients, of an assistive technology for cognition called Mobus (11, 12). We also explored if the use of Mobus could improve mnesic and executive functioning as well as autonomy of people with schizophrenia.
Methodology
Ethic
This research received ethic approval from the French General Management of Health (protocol 06 053).
Participants
Fourteen patients with SCZ according to DSM-IV criteria signed the consent form. Nine patients participated until the end of the experiment. See Table 1 for characteristics of each patient involved.
Material
 Figure 1. Main screens of Mobus. a) The patient-application displays the present time, the next activity, and allows the patient to consult his/her own list of activities (“act.”) and symptoms (“sympt.”); b) The caregiver-application allows to consult patients’ characteristics, their activities and symptoms, as well as other caregivers involved.
Figure 1. Main screens of Mobus. a) The patient-application displays the present time, the next activity, and allows the patient to consult his/her own list of activities (“act.”) and symptoms (“sympt.”); b) The caregiver-application allows to consult patients’ characteristics, their activities and symptoms, as well as other caregivers involved.Mobus provides two connected sub-applications implemented in PDA: one for the patients, the other for the caregivers (figure 1).
Patient application
Patients can either consult their list of ADL or notify the caregiver about a symptom, simply by clicking on the words “act.” or “sympt.” displayed on the touch-screen (Figure 1-a).
During an interview, the patient is said to express which ADL and symptoms he would like to record. These lists are therefore totally individualized. A color code notifies when the activity has to be performed (Figure 2-a). As soon as the patient has accomplished the current activity, he must click on the corresponding line. The click time is also recorded on a server and the ADL validation appears on the caregiver’s PDA.
ID |
Sex |
Age |
|
P1 |
F |
30 |
|
P2 |
F |
39 |
|
P3 |
F |
44 |
|
P4 |
M |
32 |
|
P5 |
F |
44 |
|
P6 |
M |
33 |
|
P7 |
M |
25 |
|
P8 |
F |
33 |
|
P9 |
M |
52 |
|
X |
M |
36 |
Persecution’s feeling |
A |
F |
35 |
Fear of technology, and a relative was hostile to the experiment |
X |
M |
30 |
Relapse |
X |
M |
28 |
Persecution’s feeling, and a relative was hostile to the experiment |
X |
M |
22 |
Relapse |
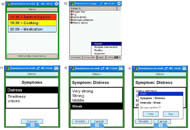 Figure 2. a) Patient's PDA : activity in red is a past activity, in yellow is a present activity, and in grey is a future activity; b) The caregiver can remotely verify if the patients validated their activities (green discs) or not (red discs). c) Patients can signal anything they are experiencing, and precise at which intensity, from anywhere, at anytime. For example, a patient signals that he is feeling distressed. d) A new window opens with some proposition of intensities. Here, the patient feels a weak distress. e) A last window opens so that the patient can verify and confirm what he selected.
Figure 2. a) Patient's PDA : activity in red is a past activity, in yellow is a present activity, and in grey is a future activity; b) The caregiver can remotely verify if the patients validated their activities (green discs) or not (red discs). c) Patients can signal anything they are experiencing, and precise at which intensity, from anywhere, at anytime. For example, a patient signals that he is feeling distressed. d) A new window opens with some proposition of intensities. Here, the patient feels a weak distress. e) A last window opens so that the patient can verify and confirm what he selected.The user has also the possibility to record what symptom is being experienced and how intense it is (Figure 2 c-d-e). This is recorded, and can be consulted by the caregiver on the server.
Caregiver application
The patients’ ADL are programmed from the caregivers’ PDAs. In collaboration with the patients, caregivers can create, modify and delete the occurrence, date and time of ADL. The caregivers can remotely verify whether the ADL have been validated or not (figure 2-b).
Variables and analysis
The rate of use of the ADL recall function was calculated by multiplying the number of ADL actually validated by the patient by 100, and dividing the result by the total number of activities initially planned. A rate of 100% corresponds to a perfect use, whereas a rate of 0% means that the patient never used Mobus to validate his activities.
Furthermore, we obtained the mean of use of the symptom function per week by reporting the number of symptoms signaled on the number of days, then multiplied by 7. We also calculated the mean percentage of use of this function in order to compare it to the rate of use of ADL recall. A rate of 100% corresponds to one use per day, so the rate could be above 100% if patients signalled more than an average of one symptom per day.
The appreciation of Mobus was obtained through a questionnaire noted on 5 filled by the patients at the end of the experiment.
On another hand, the French translation of the Independent Living Skills Scale (ILSS) (13) was used to assess the patients’ autonomy. Subjects were administered subtests of the CAmbridge Neuropsychological Test Automated Battery (CANTAB) (14, 15): Paired Associate Learning (PAL) assesses episodic memory and learning; Stocking of Cambridge (SOC) assesses spatial planning and motor control; Strategy Working Memory (SWM) assesses working memory and strategy use. Finally, the participants’ symptomatology, their quality of life and self-esteem were assessed.
Paired sample t-tests were conducted in order to compare mean scores before and after the use of Mobus. Fisher’s correlations were calculated in order to investigate the link between the use of Mobus and scores on scales.
Procedure
Interviews were organized between the experimenter, the patient and a caregiver before and after the use of Mobus. Patients were informed about the progress and goals of the experiment and signed consent forms. Mobus was presented to the patient and each function was explained and tested until the patient managed to use it without any help. Patients used their PDAs, specially customized according to their needs, during 6 weeks. Exploratory neuropsychological assessments were collected before and after the use of Mobus.
Hypotheses
More than 50% of ADL validation would reflect that patients used Mobus regularly, as well as signalling their symptoms at least one time per week. Furthermore, we expected that patients would appreciate Mobus with a score higher than 2,5/5. Finally, ADL planning would enhance the scores on neurpsychological tests.
RESULTS
 Figure 3. Bars represent the rates of use of the activity and symptom functions by the patients. The yellow line represents the scores of appreciation of the device by the users. Data ranged by increasing score of appreciation: from the patient who appreciated Mobus the less (P8) to the patient who appreciated Mobus the best (P4).
Figure 3. Bars represent the rates of use of the activity and symptom functions by the patients. The yellow line represents the scores of appreciation of the device by the users. Data ranged by increasing score of appreciation: from the patient who appreciated Mobus the less (P8) to the patient who appreciated Mobus the best (P4).The rates of use of Mobus, as well as the mean scores of appreciation by the patients, are presented in figure 3.
ADL recall
Patients validated a mean of 40% of activities. The majority (38%) were validated during the planned periods, whereas 31% and 27% were validated too soon or too late, respectively. A third of the patients used Mobus for validating more than 86% of their activities (P2, P4 and P6).
Symptom notification
Patients signalled a mean of 1,01 symptom per week. In total, they used this function with a mean of 14,44% of the time. Two patients used it more than average (P1 and P9). Four patients notified only one symptom, on one occasion. Only one patient never used the function (P4). Sixty-two percents of the symptoms were signalled after noon.
Appreciation
Patients declared that they appreciated Mobus with a mean level of 2,27/5. Among the three patients who scored above this mean, P4 awas an high users of “ADL recall” and P9 was an high user of “Symptom notification”, but no significant correlation was found between the scores of appreciation and the rates of ADL validations nor the mean notification of symptoms per week.
Exploratory results
Significant enhancement between Baseline and Endpoint was found for the “Food” subscale of the ILSS (t(8) = 2.32, p = .049). Furthermore, a significant decrease in the mean initial thinking time for the SOC subtest of the CANTAB appeared (t(8) = 3.67, p = .006), which means that performance on this test was enhanced. There were no significant improvements on other exploratory variables.
DISCUSSION
Limits
Through Mobus, patients are supposed to be permanently connected to their caregivers. This is aimed at avoiding isolation, and providing reassuring environment to the patient. Nevertheless, some schizophrenia patients can experience paranoïd symptoms (which belong to positive symptomatology). When patients strongly presented this symptom, we observed that it was difficult, indeed impossible, to ask them to use Mobus. On another hand, the fact that patients validate their ADL does not mean that they actually did them. Neverheless, it would be unethical to watch the patients, or to force them to complete their activities.
Mainly, the PDAs experienced connection breakdown. Consequently, some tasks were not updated, and sometimes, patients could not validate an activity, because they did not see it on their diary. This certainly limited the appreciation of the device. Moreover, we had hoped for more improvement on neuropsychological assessments. The connection problems probably explain this lack of results.
Hopes
Despite the technical problems, a third of the patients used Mobus for validating more than 86% of their activities. Furthermore, when they did use Mobus, most of the patients validated their ADL during the planned periods. This is a promising indication of potential effective use of Mobus in the future. On another hand, some patients had few activities (social avoidance, apragmatism): planning ADL with an entertaining tool could have encouraged them to plan new occupations. For example, P2 took steps to register for a bicycle course, P7 participated in a soccer course, and P3 spent more time in her own apartment (gradually leaving parents’ home). Overall, the PDA personalization was very important, because of the various degree of autonomy and occupation of patients. Finally, some patients reported that they used “ADL recall” at the beginning, but after several days, they began to habituate to the repeated activities and remembered them without reminders. This encouraging result represents the potential some patients will gain in their ability to learn and organize their activities through use of Mobus.
The fact that symptoms seem to be experienced mainly after noon could be taken into account in the treatment by caregivers. Interestingly, the highest users of “ADL recall” (P2, P4 and P6) were not the same people as the highest users of “Symptom notification” (P1 and P9). Patients may find it difficult to use both functions. However, this explanation does not correspond to the reports of the patients themselves, who found the application easy to use. Thus, it needs to be verified without connection problems. In fact, some patients may have used the “Symptom notification”, which did not encounter any problems, rather than the “ADL recall” which did not update. On the other hand, some patients used the “ADL recall” function despite the technical problems and were also less motivated to use the “Symptom notification”. Finally, patients could have been more motivated to improve their autonomy than to report their symptoms. Knowing that patients with schizophrenia have difficulties recognizing their illness, we changed the name of this function in the next study, in order to motivate more patients to use it. The name “Experiences” would be better, as it would avoid focusing on symptoms.
It is worth noticing that the subjective appreciation of the device seems to be in contradiction with its use. For example, P1, P2, and P6 had a weak appreciation of Mobus whereas they noticeably used it. Thus, if the connection problem was the core factor of weak appreciation, it did not prevent patients from using the device. We also can hopefully imagine that, with a good functioning of Mobus, most of patients would use it, but also appreciate it. As a matter of fact, the results of P4 show that it is possible, with 100% of validated ADL and a total appreciation of the device.
Finally, the significant enhancement on the score at the “Food” subscale of the ILSS could mean that, at the end of the experiment, patients performed better at tasks related to food. On another hand, significant decrease was found on the mean subsequent thinking time of the SOC. This could reflect that our procedure might have led to an improvement in executive functioning, as we expected. Nevertheless, these results must be replicated with more subjects and a control group.
Solution
In sum, programmers detected that new connection problems arose from mismatches in the programming code. Thus, they reshaped the entire underlying program which is now much more stable and connection problems have been repaired.
CONCLUSION
Mobus provided ecological data that could be valuable for clinical follow-up of schizophrenia patients. This pilot study was useful to point out the pitfalls of the technology in its application on cognitive rehabilitation. We are currently testing the enhanced version of Mobus in a randomised controlled study involving 2 groups of 34 schizophrenia patients.
REFERENCES
- Green, M.F. (1996). What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 153(3): p. 321-330.
- Bowie, C.R. and P.D. Harvey. (2005). Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatric Clinics of North America. 28(3): p. 613-633.
- Chambon, V., et al. (2008). The architecture of cognitive control in schizophrenia. Brain. 131(4): p. 962-970.
- Norman, R.M., et al. (1999). Symptoms and cognition as predictors of community functioning: a prospective analysis. Am J Psychiatry. 156(3): p. 400-5.
- Cole, E. (1999). Cognitive prosthetics : An overview to the method of treatment. NeuroRehabilitation. 12: p. 39-51.
- LoPresti, E., A. Mihailidis, and N. Kirsch. (2004). Assistive technology for cognitive rehabilitation : State of the art. Neuropsychological Rehabilitation. 14(1/2): p. 5-39.
- Sablier, J., E. Stip, and N. Franck. (2009). Cognitive remediation and cognitive assistive technologies in schizophrenia. L'Encephale. 35: p. 160-167.
- Wilson, B.A., et al. (2001). Reducing everyday memory and planning problems by means of a paging system: a randomised control crossover study. Journal of Neurology, Neurosurgery and Psychiatry. 70(4): p. 477-82.
- Wilson, B.A., et al. (1997). Evaluation of NeuroPage: a new memory aid. Journal of Neurology, Neurosurgery and Psychiatry. 63(1): p. 113-5.
- Davies, D.K., S.E. Stock, and M.L. Wehmeyer. (2002). Enhancing independent time-management skills of individuals with mental retardation using a Palmtop personal computer. Mental Retard. 40(5): p. 358-365.
- Giroux, S., et al. (2008). Enhancing a mobile cognitive orthotic: A user-centered design approach. International Journal of ARM. 9(1): p. 1-12.
- Sablier, J., et al. (2007). Study on the convivial use of an electronic agenda by individuals with schizophrenia. Santé Mentale au Québec. 32(2): p. 209-224.
- Cyr, M., et al. (1994). Assessment of independent living skills for psychotic patients. Further validity and reliability. Journal of Nervous and Mental Disease. 182(2): p. 91-97.
- Levaux, M.N., et al. (2007). Computerized assessment of cognition in schizophrenia: promises and pitfalls of CANTAB. Eur Psychiatry. 22(2): p. 104-15.
- Stip, E., L. Lecardeur, and A. Ali-Sepehry. (In Press 2008). Computerized assessment of neurocognition in schizophrenia: An exploratory meta-analysis of CANTAB findings. European Psychiatric Review.
Aknowledgements
We want to address grateful thanks to Francis Bouchard, Nicolas Marcotte and Jean-Paul Viboud and all the involved patients and caregivers of the psychiatric hospital “Le Vinatier” in Lyon (France). This research was made possible thanks to grants from the scientific committee of research of the “Le Vinatier”, the Rhône-Alpes region, the Canadian Eli Lilly Chair of schizophrenia research and the Egide association from French ministry of foreign affairs.
FIRST AUTHOR
Juliette Sablier
Center of Cognitive Neuroscience
67 Boulevard Pinel
69675 Bron Cedex
01133437911220