Carmen P. DiGiovine1,2, PhD ATP RET, Richard M. Schein3,4, PhD, MPH, Mark R. Schmeler3,4 PhD, OTR/L, ATP
1. The Ohio State University
2. The Ohio State University Medical Center
3. The University of Pittsburgh
ABSTRACT
The selection of appropriate assistive technology (AT) devices is critical to matching the technology to the individual given the activity and environment. This increases utilization and decreases likelihood for abandonment. In order to facilitate this process, a mechanism is developed and implemented for reviewing technology and sharing information. The process is based on the work of Batavia and Hammer (Batavia & Hammer, 1990). The mechanism includes reviewing the devices based on seventeen factors, a list of both indications and contraindications as well as a device summary. Twenty-one reviews were generated with input from 15 clinicians and posted in a blog format on a secure internet browsing site. The system seamlessly integrates into clinical practice providing clinicians with the information they need to provide appropriate technology based on current evidence- based practice.
INTRODUCTION
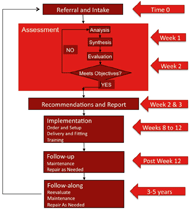 Figure 1: Assistive Technology Service Delivery Process – modified (Cook & Polgar, 2008; Szeto, 2001). d
Figure 1: Assistive Technology Service Delivery Process – modified (Cook & Polgar, 2008; Szeto, 2001). dThe assistive technology (AT) service delivery process has been well documented in the literature (Cook & Polgar, 2008; Szeto, 2001). The iterative nature of the process is displayed in Figure 1. AT models within which the process is conducted includes the HAAT, SETT, PHAATE, and ICF (Cook & Polgar, 2008; Cooper, 2007; World Health Organization, 2002; Zabala, 2005). For example, the PHAATE model is displayed in Figure 2. A key component of the models and the AT service delivery process is the ability to match the appropriate technology to the individualistic requirements of the individual. Lenker and Paquet proposed a new conceptual model for AT research and practice (Lenker & Paquet, 2004). In their model, the intention of an individual to continue using an AT device is based on the relative advantage of the device in performing a task in comparison to parallel interventions [Figure 3]. Therefore, it is critical not only to match the AT device to the individual characteristics at time one, but also to ensure the match continues in a longitudinal fashion. A mechanism does not currently exist to systematically analyze AT devices in a repeatable fashion. Given a cohort of rehabilitation professionals (e.g. OT, PT, RT, RC, RE, SE) working in the field of AT across multiple locations, a need exists to disseminate the systemic analyses of AT devices so that it is available in a just-in-time fashion. Therefore, the purpose of this project is to develop and implement a mechanism for reviewing technology and sharing information.
METHODS
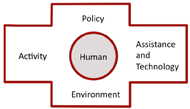 Figure 2. PHAATE Framework (Cooper, 2007) d
Figure 2. PHAATE Framework (Cooper, 2007) dA multi-step process was utilized to develop the AT device review architecture and mechanism. In terms of the device reviews, collaboration between the University of Pittsburgh and The Ohio State University performed a literature review on the topic of evaluating AT devices. Two methodologies were predominant in the literature, the criteria developed by Batavia and Hammer (Batavia & Hammer, 1990) and the criteria developed by The Center for Universal Design at North Carolina State University (Story, Mueller, & Mace, 1998). The criteria developed by Batavia and Hammer was selected based on input from a set of clinicians at four VA-Polytrauma Rehabilitation Centers (PRC) [Table 1]. The criteria provided the basis for all reviews. A second component of the review process, which was encouraged by the clinicians, was the inclusion of a numerical scale for each criterion. The scale selected was a 5-point likert scale ranging from “not satisfied at all” to “very satisfied”. The final component of the review, which was a direct result of input from the clinicians at the PRCs, was the inclusion of a device summary, a description of indications for device utilization and a description of contraindications for device utilization. The summary, indications, and contraindications are intended as an introduction to the review, and typically are each 1 to 2 paragraphs in length. The collaborative process among the clinicians from the PRCs and consultants from the University of Pittsburgh and The Ohio State University, created an AT Device review with three distinct components: an introduction including the summary, indications and contraindications; a review including 17 criteria; and a likert scale to quantitatively describe the AT device for each criterion.
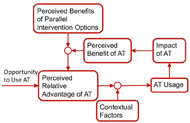 Figure 3. Conceptual model for AT Outcomes (Lenker & Paquet, 2004) d
Figure 3. Conceptual model for AT Outcomes (Lenker & Paquet, 2004) dAffordability |
Learnability |
Compatibility |
Operability |
Consumer Repairability |
Personal Acceptability |
Dependability |
Physical Comfort |
Durability |
Physical Security |
Ease of Assembly |
Portability |
Ease of Maintenance |
Securability |
Effectiveness |
Supplier Repairability |
Flexibility |
Hammer, 1990) and the criteria developed by The Center for Universal Design at North Carolina State University (Story, Mueller, & Mace, 1998). The criteria developed by Batavia and Hammer was selected based on input from a set of clinicians at four VA-Polytrauma Rehabilitation Centers (PRC) [Table 1]. The criteria provided the basis for all reviews. A second component of the review process, which was encouraged by the clinicians, was the inclusion of a numerical scale for each criterion. The scale selected was a 5-point likert scale ranging from “not satisfied at all” to “very satisfied”. The final component of the review, which was a direct result of input from the clinicians at the PRCs, was the inclusion of a device summary, a description of indications for device utilization and a description of contraindications for device utilization. The summary, indications, and contraindications are intended as an introduction to the review, and typically are each 1 to 2 paragraphs in length. The collaborative process among the clinicians from the PRCs and consultants from the University of Pittsburgh and The Ohio State University, created an AT Device review with three distinct components: an introduction including the summary, indications and contraindications; a review including 17 criteria; and a likert scale to quantitatively describe the AT device for each criterion.
| Apple iPod Touch | Lithographica and SmallTalk |
| Kurzweil 3000 | PredictAble |
| Read & Write Gold 9 | TopEnd Force2 |
| DynaVox Xpress | WordQ / SpeakQ |
| Quartet ECU | NeuroSwitch |
| Livescribe Pulse | Mount’n Mover |
| Apple iPad | SAJE Roomate Plus ECU |
| DynaVox DynaWrite | iPad vs iPad2 |
| AssistiveWare Proloquo2Go | Primo Possum |
| Chat PC Silk+ | Inspiration |
| Windows 7 |
The second step in the process was developing a platform to disseminate the reviews, and allow other clinicians to continually provide input on the reviews as they get more experience with the device. A platform based on Microsoft SharePoint was selected for disseminating the information. The SharePoint platform is utilized within the Department of Veterans Affairs, The University of Pittsburgh, and The Ohio State University. Therefore, the clinicians and consultants are familiar with the platform. Furthermore, the platform is run through a web browser, so it doesn’t require installing software on the individual computers. Therefore, clinicians will have access to the information as long as they have access to a computer with an Internet connection. As part of the overall project with the Department of Veterans Affairs, a secure clinical portal was created based on the SharePoint platform for the dissemination of information among all stakeholders. One section of the portal incorporated a blog to house the device review, while allowing comments and questions from other clinicians. A key criterion in the dissemination process was the ability to post the review and allow for other individuals to provide input so that the review becomes a living document. While allowing for input, editing original posting is limited to the authors and editors of the review. The utilization of a blog format within the clinical portal provides a simple mechanism for disseminating reviews, commenting on the reviews over time, and accessing the reviews in order to match the features of AT to the unique requirements of the individual with a disability.
The final step in the process was determining a process for reviewing the AT Devices, and determining specific devices and review order. The goal of the project was to review 18 AT devices over an 18-month period. The reviews were broken into 3 sets of 6. The first set of 6 were completed by the consultants from The University of Pittsburgh and The Ohio State University; second set completed in collaboration between the consultants and the clinicians at the PRCs; and the third set completed by the clinicians at the PRCs with editorial support from the consultants. The specific devices were selected based on input from the individual clinicians. An editable list of devices that had been reviewed and were up for review, as well as the site, which was responsible for the review was maintained on the clinical portal. Therefore, the clinicians had a mechanism to easily check for past reviews, as well as request future reviews. The mechanism described above provides a method for initiating the device review process, training clinicians on the process for device review, and requesting future reviews.
RESULTS
Twenty-one AT device reviews were completed over a 21 month period [TABLE II]. Six of the reviews were AAC devices or software, six were computer access hardware or software, four were electronic cognitive devices, three were electronic activity of daily living devices, one was recreational technology and one was mounting hardware. Excerpts from an example AT device review are found in the Figure IV.
The generation of reviews typically occurred in an iterative process. A primary author was identified and wrote the draft review. Secondary authors then edited the content and provided additional content. Finally, the editors reviewed the content, and if any changes were necessitated, they consulted with the authors prior to making any changes. This process of including primary and secondary authors, in conjunction with editors, provided a mechanism with low overhead requirements on any one individual, while providing oversight.
DISCUSSION
AT Device reviews are a critical component of the service delivery process. The information provided by the reviews is key to matching the unique requirements of the individual for performing a specific activity in a specific environment to a specific device. Numerous reviews exist in journal articles, magazine articles, websites, list-serves, and discussion boards. However, a systematic process for reviewing individual AT devices within a clinical setting with multiple professionals in multiple locations has not been described in the literature.
 Figure IV: Excerpt of AT Device Review d
Figure IV: Excerpt of AT Device Review dOne of the overarching goals in the development of an AT program is the inclusion of outcome measures as part of the feedback system to determine not only the success of the service delivery process on an individual basis, but also on a programmatic basis. Process engineering directly addresses the key components of the process, and identifies mechanisms to the process that are either incomplete or ignored. One of those processes was a mechanism for reviewing AT devices for potential utilization by individuals with disabilities.
There are numerous pros and cons to the process that was put into place. The review process provides a mechanism with low overhead for each review. The criteria are well defined and have standard questions for each criterion to lead the clinician in reviewing the device. The goal was to minimize the verbiage for each criterion in order to make it easy to write the review. Furthermore, the review process allows for reviewing categories of devices (e.g. mechanical switches), or reviewing previous models. This creates a historical record of reviews that can be accessed by clinicians throughout the assessment process.
The review process not only assists the clinician in reviewing a single device, but also helps them categorize the important features of other devices, regardless if a formal review has been conducted.
The clinical portal is conducive to providing information that is always available in a single location, and is conducive to updating information as more clinicians get experience with the device. The portal allows clinicians to provide their experience with the device across multiple settings and locations.
Conversely, the reviews are not exhaustive and do not require incorporating specific technical data from each AT device. This was intentional, as manufacturers typically provide this information in the product literature.
Furthermore, not every criterion applies to each device, which can lead to confusion when performing the review.
The development of the AT labs within the VA-PRC has focused on utilizing the ICF as an overarching framework. Within that framework, devices are defined under the environmental factors and the reviews are critical to identifying devices for trial purposes and eventual recommendation and implementation.
CONCLUSION
The key to successfully utilizing this information in clinical practice is to create a system that seamlessly integrates into the service delivery model, and is available for clinical utilization. This is consistent with developing procedures that build on the clinician’s expertise as part of evidence-based practice. The development of the model is based on the AT evaluation criteria developed by Batavia and Hammer along with universal design principles. In order to insure that the evaluation model met the clinicians’ requirements, consultants field tested and held focus groups to answer any questions, verify requirements, and disseminated results back to all personnel involved.
REFERENCES
Batavia, A. I., & Hammer, G. S. (1990). Toward the development of consumer-based criteria for the evaluation of assistive devices. J Rehabil Res Dev, 27(4), 425-36.
Cook, A. M., & Polgar, J. M. (2008). Introduction and framework. Cook & Hussey's assistive technologies: Principles and practice (3rd ed., pp. 3-33; 1). St. Louis, MO: Mosby, Inc.
Cooper, R. A. (2007). Introduction. In R. A. Cooper, H. Ohnabe & D. A. Hobson (Eds.), An introduction to rehabilitation engineering (pp. 1-18). Boca Raton, FL: Taylor and Francis.
Lenker, J. A., & Paquet, V. L. (2004). A new conceptual model for assistive technology outcomes research and practice. Assist Technol, 16(1), 1-10.
Story, M. F., Mueller, J. L., & Mace, R. L. (1998). The universal design file: Designing for people of all ages and abilities. Raleigh, N.C.: NC State University, The Center for Universal Design.
Szeto, A. Y. J. (2001). Rehabilitation engineering and assistive technology. In J. D. Enderle, S. M. Blanchard & J. D. Bronzino (Eds.), Introduction to biomedical engineering (pp. 905-941). New York: Academic Press.
World Health Organization. (2002). Towards a common language for functioning, disability and health. Geneva, Switzerland: World Health Organization.
Zabala, J. S. (2005, February / March 2005). Ready, SETT, go! getting started with the SETT framework. Closing the Gap, 23
ACKNOWLEDGEMENTS
This work was supported by the Department of Veterans Affairs as part of the Assistance and Advisory Services for Polytrauma Rehabilitation Center Assistive Technology Labs. Also we would like to thank the clinicians at the PRC AT Technology Labs for sharing their insights on the development and performing AT reviews.