А. E. Khizhnikova1, A.S. Klochkov1, N. A. Suponeva1
1Research Center of Neurology, Moscow, Russian Federation;
INTRODUCTION
A promising but insufficiently studied method is virtual reality (VR), as well as its combination with other techniques like arm weight support training. Motor training in virtual reality (VR) with arm weight support creates the necessary facilitated environment for motor skills relearning [3].
MATERIALS AND METHODS.
45 patients (27 males and 18 females) with medium age 55 [45;65] years were enrolled in this study. All patients had one supratentorial lesion due to ischemic or hemorrhagic stroke (confirmed by MRI). Medium stroke age was 7 [4;12] months. All patients had moderate to severe upper limb paresis measured by Medical Research Council Scale for Muscle Strength and Fugl-Meyer assessment of physical performance (FMAS) upper extremity subscore 45 [35;55]. All patients received 2 weeks of a rehabilitation course, 5 days per week, 45 minutes daily.

The control group (n=20) received conventional therapy sessions with arm weight support (a system of pulleys), visual feedback (via mirror) and comparable set of tasks – reaching, grasping, manipulating objects.

RESULTS.
|
Before rehabilitation | After rehabilitation | p-level |
| Moderate paresis, Ме [25%;75%] | 1,5 [1,24; 1,71] | 1,26 [0,9; 1,62] | p=0,045 |
| Severe paresis, Ме [25%;75%] | 2,25 [1,65; 3,76] | 2,66 [1,11; 3,05] | p=0,043 |
| Normal, Ме [25%;75%] | 0,96 [0,87; 1,16] |
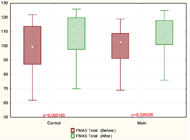
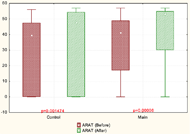
In our study, the clinical assessment (FM and ARAT scales) showed that paretic hand recovery was found more in patients with moderate and severe paresis. Statistically significant improvements in the arm motor function (FMAS) were found in both groups. However, subsection analysis revealed that the patients of the main group compared to the control group had a more significant improvement in wrist movements. In ARAT was found that in patients with moderate paresis significant improvements occur in both main and control groups. In patients with severe paresis, improvements were observed only in the main group.
However, after motion analysis, a different stereotype of movement recovery was found in different groups of patients. In patients with severe paresis, an increase in the deviation of the movement pattern from the physiological movement was observed. At the same time, the normalization of the motor pattern was noted in patients with moderate paresis.
| Movement | Before rehabilitation, Ме [25%;75%] | After rehabilitation, Ме [25%;75%] | p-level |
| Elbow extension | 124 [116;126] | 112 [109; 125] | 0,01 |
| Shoulder flexion | 36 [27; 41] | 21 [20; 32] | 0,02 |
| Shoulder abduction | 10 [10; 17] | 19 [18; 22] | 0,04 |
| Velocity shoulder abduction | 17 [13; 20] | 48 [39; 65] | 0,02 |
| Velocity elbow extension | 39 [26; 69] | 29 [18,39] | 0,02 |
The time of reaching test execution in patients with severe paresis after rehabilitation was longer than before and exceeded the normal time more than twice. Curiously, these changes in patients with severe paresis were associated with an increase in functionality in the paretic arm (p>0,05).
The kinematic parameters such as elbow extension, shoulder abduction and angular velocity in shoulder and elbow joints after rehabilitation were worsened. After a rehabilitation course was founded decreasing of the angular velocity of the elbow joint extension, increasing of the angular velocity of the shoulder joint, decreasing of the flexion in the shoulder joint and angular speed of the elbow joint extension.
The analysis of trunk movements in severe paresis patients was shown that after rehabilitation course the trunk compensatory strategy was increased (trunk was mowed forward when patient reach the glass). These changes were associated with an increase in functionality in the paretic arm (p>0,05).
CONCLUSIONS.
|
Shoulder displacement |
Before rehabilitation, Ме [25%;75%] |
After rehabilitation, Ме [25%;75%] |
Healthy shoulder |
23 [19,8; 57,44] |
66 [49;81] |
Paretic shoulder |
169 [88; 178] |
215 [162; 229] |
If we summarized data of clinical and biomechanical parameters we see, that patients with severe paresis formed the new compensatory strategy of motion. Because of the significant changes in functional recovery are combined with worsened of biomechanical parameters.
It is believed that it is the resistance to pathological synergies and the forced training in physiological movement is the most effective method. However, correction of pathological synergies allows developing the most energy-efficient stereotype of movements for patients with regard to their individual capabilities. Combined VR and weight support training can be more effective to restore the impaired motor function after stroke than conventional weight support training. This approach contributes to the motor pattern reorganization through biomechanical and visual feedback, projected into the virtual space.
REFERENCES
[1] Beebe J.A., Lang C.E. Active range of motion predicts upper extremity function 3 months after stroke. Stroke. 2009 40 (5): 1772–1779.
[2] Cirstea M.C., Levin M.F. Compensatory strategies for reaching in stroke. Brain. 2000 123 (5): 940–953.
[3] Laver K.E., George S.,J.E. Thomas, M. Deutsch. Crotty Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2015 12 (2): 83.