I. V. Saenko2, А. E. Khizhnikova1, N. A. Suponeva1, L. A. Chernikova1
1Research Center of Neurology, Moscow, Russian Federation; 2Institute of Medico Biological Problems, Russian Academy of Sciences, Moscow, Russian Federation
INTRODUCTION
The mechanisms underlying the locomotion recovery in poststroke patients remain unknown. Regent Multi-modal Complex Exoskeleton is used in Russia to improve walking in neurological patients over the past few years. Its positive effects on clinical data are known. However, its influence on reorganization of cortical zones involved in control walking is not entirely clear [1-3].
MATERIALS AND METHODS.
We examined 14 patients aged 50.3 [49; 62] years who had suffered an ischemic stroke in the basin of the middle cerebral artery and had mild to moderate paresis of a leg; the mean leg paresis severity was 22.2 [17.0; 27.0] by the Fugl–Meyer scale (a normal score is 34). The stroke was subcortical in all of the patients and affected the right hemisphere in seven patients and the left hemisphere in the other seven patients. The stroke history varied from 1 to 28 months and exceeded 6 month in 78.6% (11 out of 14) of the patients. On average, the stroke history was 14.2 [7.0; 23.0] months. All of the patients were able to walk 10 meters without any assistance.
An examination was performed before and after rehabilitation and included the following tests:
- fMRI in the passive sensorimotor paradigm imitating the support loading during slow walking
- analysis of functional connectivity of the fMRI- identified zones of interest.
We used a passive sensorimotor fMRI paradigm developed previously to imitate the support loading during walking. A support afferent flow was stimulated using a Korvit support loading stimulator. The Korvit device makes it possible to reproduce the physiological pat- terns of support responses arising during locomotion. The device uses the pneumonic cells to create pressure on the plantar support zones, which function following the actual locomotion pattern. With each subject, a paradigm (test) used in one scanning session was trained before the study in the scanning room. The paradigm was designed in blocks and included 54 series, eight blocks with seven measurements each, four active and four passive tasks, each lasting 26.25 s (rounded up to 27). The total test lasted 3.5 min, TR = 3.75 s.

MRI study employed MRI tomograph with a magnetic field strength of 1.5 T (Siemens MAGNETOM Avanto).

Apart from conventional rehabilitation, the patients with a past ischemic stroke received kinesiotherapy with a MCE (Multimodal Complex Exoskeleton “Regent”) - ten sessions, five times a week.
A system of elastic loading elements (ELEs) is incorporated in the MCE to reproduce the muscle topography distribution and to provide not only an axial (vertical) load on trunk and leg muscles, but also postural changes, including necessary angles at large joints, flexion and extension, and trunk rotation. The mechanism of action of the suit is presumably based on correcting a proprioceptive flow from joints, tendons, and muscles via a system of ELEs, which are arranged according to the antigravitational muscles topography of the trunk and limbs (antagonist muscles) to provide for a correction of distorted locomotor acts. The ELEs were stretched to ensure a correct position of the lower limbs and to normalize walking movements. The positions of the shoulders and trunk were corrected using waistcoat ELEs. Postural asymmetry was substantially reduced by this means.
RESULTS.
Activation zones identified by fMRI before rehabilitation with the MCE: the left inferior parietal lobule ({IPL), right inferior parietal lobule {(IPL), primary sensorimotor and supplementary motor cortical areas (SM1 + SMA) |
Activation zones identified by fMRI after rehabilitation with the MCE: the left inferior parietal lobule ({IPL), right inferior parietal lobule ({IPL), primary sensorimotor and supplementary motor cortical areas (SM1 + SMA). |
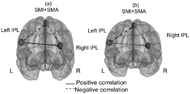
Functional connectivity data (a) before and (b) after rehabilitation with the MCE. SM1 + SMA, primary sensorimotor and supplementary motor areas; left IPL, left inferior parietal lobule; right IPL, right inferior parietal lobule; L, left (healthy) hemisphere; R, right (affected) hemisphere.
Figure 4. Functional connectivity data
In our study a processing of group data identified the primary sensorimotor area and supplementary motor areas (SM1+SMA), and inferior parietal lobules (IPL) on the right and left as sensorimotor cortical activation zones by fMRI before a course of MCE training. After a course of MCE training, we observed a decrease in activation zone of the IPL, especially in the healthy hemisphere, and an excessive increase in activation zone in the SM1+SMA areas. Changes in functional connectivity were revealed in special analyses performed before and after a rehabilitation course. Namely, activating interhemispheric connections between the secondary associative areas grew weaker, and a positive connection arose between the SM1+SMA areas (motor regions) and the IPL (an associative somatosensory area) in the affected hemisphere.
CONCLUSIONS.
To summarize, our study showed that the activation zones detectable by fMRI indicatives of positive changes in the neuroplastic processes; i.e., the activation zones decreased in the secondary sensory areas (the inferior parietal lobules) and increased in the primary sensorimotor and supplementary motor areas. Changes in the functional connectivity were revealed in the special analyses performed before and after a rehabilitation course. Namely, the activating interhemispheric connections between the secondary associative areas grew weaker, and a positive connection arose between the primary sensorimotor and the supplementary motor areas (motor regions) and the inferior parietal lobule (an associative somatosensory area) in the affected hemisphere. The findings can be interpreted as a decrease in inhibitory influences from the associative somatosensory area on the motor areas in the affected hemisphere. A more exact interpretation of the findings needs further studies with a larger subject sample and an effective connectivity method.
|
BEFORE | AFTER | ||||
ROI |
X,mm | Y,mm | Z,mm | X,mm | Y,mm | Z,mm |
|---|---|---|---|---|---|---|
| IPL left | -59 | -35 | 27 | -42 | -27 | 23 |
| IPL right | 38 | -31 | 20 | 41 | -31 | 22 |
| SMl+SMA | 3 | -32 | 66 | 3 | -35 | 67 |
REFERENCES
[1] Iseki K., Hanakawa T., Shinozaki J., Nankaku M., Fukuyama H. Neural mechanisms involved in mental imagery and observation of gait. NeuroImage. 2008 41: 1021– 1031.
[2] a Fougere C., Zwergal A., Rominger A., Forster S., Fesl G., Dieterich M., Brandt T., Strupp M., Bartenstein P., Jahn K. Real versus imagined locomotion: A [18F]-FDG PET- fMRI comparison. NeuroImage/ 2010 50: 1589-1598.
[3] Bakker, M., De Lange, F. P., Helmich, R. C., Scheeringa, R., Bloem, B. R., and Toni, I. Cerebral correlates of motor imagery of normal and precision gait. NeuroImage. 2008 41, 998–1010.
This work was supported by the Russian Foundation for Basic Research (project nos. 16-29-08209 ofi _m).