Sennett Yang, Kyle Nicholson, Jesse Shen
Students Participating in Biomedical Senior Design
slyang@wustl.edu, kylenicholson@wustl.edu, j.shen@wustl.edu
Undergraduates at Washington University in St. Louis
Need statement
A way to alert clinicians of trachea tube removal in children before an oximeter triggers an alert so that someone can restore the airway before permanent damage occurs.
Project Scope
Tracheostomy tubes help children with respiratory difficulties to breathe and are vital in getting oxygen into the body. With child patients, accidents happen involving the pulling of the tracheal tube out of its socket, cutting off the flow of oxygen from their bodies instantly. This project aims to design a device that alerts physicians earlier than current detection method when an endotracheal tube is removed. Due to the silent nature of hypoxia, clinicians often fail to see the problem until the patient's vitals on an oximeter measure blood oxygen levels that show distress, requiring a fast response. Some project considerations not immediately related to earlier detection include Ranken Jordan's focus on providing mobility to their patients and the focus on young patients, requiring smaller sized, aesthetically soothing, and durable solutions. A prototype that meets these needs on a simulated patient (e.g. a life-size baby doll) is the desired end result of this project.
Background of the Problem
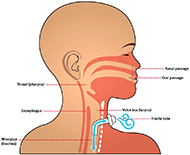
Tracheostomy tubes serve as a vital connector of air for patients that have trouble breathing and have had tracheostomies. Figure 1 graphically depicts how endotracheal tubes effectively serve as an upper respiratory tract. The device being designed will likely be closely associated with tracheostomy tubes being used with patients. Based on visits to Ranken Jordan, the environment that the proposed device will be working in will be a sterile hospital bedside. The device may operate at the interface between patient and tubing, potentially in the upper respiratory tract or near the skin.
The device proposed will serve to only alert physicians, not to prevent decannulation, the process of removing a medical tube-- in this case referring to a Tracheostomy tube. This is because there are devices on the market that serve as fail safes, but there are not as many alarm systems. Immediate care is given to patients as caregivers are available round the clock at Ranken Jordan so realizing decannulation has occurred is the primary challenge faced. Furthermore, the procedure is relatively simple and can be done rapidly. (O'Connor & White)
The end product of this project will be catered to the needs of Ranken Jordan, which accepts patients until their 21st birthday. Tentatively, this narrows the population for this project to child patients with endotracheal tubes as child versions of these tubes differ in dimensions (Equipment sizing chart). Due to the custom-order nature of this project, it may prove an attractive product to other intensive care children' hospitals with similar needs. As the end product serves as a preventative measure, its coverage of the population will be high. Approximately 20 million endotracheal tubes are sold in the U.S. every year (ENOX LTD). In terms of accident prevention, one source has found that accidental endotracheal removal from patient error and machine entanglement numbers approximately 10% in adults in intensive care units of a tertiary 650-bed hospital (Carrión et al.). This number is likely higher in children who are free to move in their hospital beds meaning at least thousands of fatal accidents may be prevented every year assuming full adoption of this device.
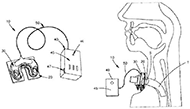
One such detection method is the use of circuitry and alarm systems that engage with the tube to detect accidental decannulation (Figure 2). The design of this patented device includes a sensor that is attached to the tracheal tube coupled with an actuator. The actuator is made of at least one magnet that produces a magnetic field and is sensed by the sensor. This sensor acts as a magnetic switch which triggers the alarm system in response to receiving a signal (Owens).
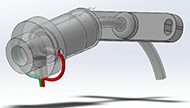
Another device, called TrachAlert (Figure 3), although not specifically used for the detection of accidental decannulation, was developed to monitor younger patients. The TrachAlert system detects breathing patterns and obstructions or abnormalities. The system is added onto the protruding portion of the tracheal tube without obstruction of cannulation. (Stevens Institute of Technology 2019).
The current detection method at Ranken Jordan is by oximeter alarm. All patients are fitted with an oximeter lead that sends a signal to their vital monitor and is constantly connected to the patient and reports to the nursing station and a readout next to the patient's bedside. This alarm has a delay between the removal of a tracheal tube and the patient's blood oxygen levels dipping below the set critical value for an alarm. Another available method for detecting tracheal tube malfunctions and removal are present in some ventilator models that use pressure sensors to monitor dramatic and abnormal changes in pressure in the trachea resulting from blockages or removal of the tracheal tube. This method of detection was not found during preliminary meetings about the problem of accidental decannulation but is a good method of detection to compare any proposed solution to. (Rady Children's Specialists, 2010).
FDA Regulation
According to the Federal Food, Drug and Cosmetic Act enforced by the FDA, the sticker and circuitry product are defined as a medical device because it is intended to mitigate a condition or disease in humans. The sticker is being used to detect decannulation of a tracheostomy tube, thereby preventing trachea collapse. Furthermore, this product is defined as a class II medical device because it requires post-market surveillance and special controls beyond the general controls needed for class I medical devices such as manufacturing operations testing and labeling (Center for Devices, 2018). Specific special controls that apply to the sticker and connection cables include intended specifications, adverse event documentation for both clinical and non-clinical uses, and human factor and usability engineering and consideration. Class II devices present a more moderate risk of harm to the patient compared to minimal risk of class I devices. Due to the proximity of the sticker and circuitry to the tracheostomy tube, more moderate harm presents itself in the form of potential accident or irritation. Caretakers would be required to monitor circuitry to prevent malfunction or adverse situations to the patient such as irritation or injury. Manufacturers of this medical device would be required to register themselves through the FDA while the product itself is being listed. Before the product can be commercially mass distributed, a premarket notification 510(k) must be submitted. This document demonstrates that this product is substantially equivalent to another device that is commercialized in the United States. This device is called the predicate devices; comparison to the predicate should show a shared intended use and technological characteristic. The predicate device found to test against is a medical safety tube device that also consists of an alarm circuitry and a sensor that transmits a signal to the alarm if a break is detected. The sensor is also located near the tube and circuitry is comprised of a magnetic connection (Medical Safety Tube Device, 2017).
Once the device is approved as a class II device, it is possible that this device would be used in clinical studies in an investigational device exemption, but most class II devices are exempt from these studies. The final parts of the process are included as general controls such as quality system regulation, which includes an FDA inspection of methods of manufacturing and distribution, labeling of the device, and reporting any incidents in which the device causes injury or accident.
Description of Final Approach and Design

At the time writing, we have narrowed down the design of the device to two similar options. They both involve a conductive fabric sticker connected to cables that will sense breakage of the circuit as seen in Figure 4. The sticker uses kinesiology tape to connect to the patient's neck surrounding the tracheal tube while the connection cables are attached to the tracheal tube itself.
The difference between the two designs is their method of connection: one uses magnets sewn into the fabric and soldered onto the cables, and one uses conductive hook and loop. We were in the process of deciding between the two as the hook and loop could potentially attach very strongly while the magnets were easily disconnected. The deciding factor was going to be our own tests to see how tracheal tubes are decannulated as we could not find literature on the subject.
Outcome (Results of any outcomes testing and/or user feedback)
The device delivers on being an alarm system that detects disruption between the trach tube and the patient quickly. When the sensor is tripped, the alarm sounds within seconds. It also appears to be consistent in its accuracy. Plans to simulate with patient models were also in the pipeline to ensure factors in detecting trach tube removal could be accounted for.
In the future, this device could be greatly improved in its usability. We would seek to minimize its size and weight to allow for patient mobility as the client hoped. Some issues we are already aware of include alarm volume and clinical convenience of the device. The power system could be reconfigured to ensure a loud enough alarm can be delivered, and battery life could be fully integrated. Sanitization and set-up would also be better optimized on this device so that it remains an afterthought for clinicians.
Cost
Our budget for the prototype was $180 and included, kinesiology tape, faraday fabric, wires, magnets, hook and loop fabric, and an arduino starter kit. The cost to produce a single prototype was much smaller. A single arduino, wiring, speaker, two small fridge magnets, and fractions of kinesiology tape and faraday fabric are calculated below.
- Arduino- $20 for an arduino nano in bulk from Mouser Electronics. Ideally, for mass production, we would customize the circuit to eliminate unnecessary components and decrease manufacturing costs. Our estimated cost of production for the circuit board is less than $4 dollars.
- Kinesiology Tape- $10 for 20, 10in. strips of kinesiology tape on Amazon.com. Each prototype sticker required a 2in x 2in section of fabric so each prototype would cost less than 10 cents to make.
- Faraday Fabric- $20 for 43"x39" Each prototype requires a 1.5"x1.5" square, meaning that each swatch of fabric would yield 728 stickers, costing less than 3 cents to make.
- Magnets- $10 for 300 magnets on Amazon.com. If we use two for each sticker, the cost to use the magnets is less than 7 cents.
- Wire- $8 for 100ft on Amazon.com. If we use 2ft conservatively on each device including scrap, the cost to use wire with this source is 16 cents.
The manufacturing cost for a device would likely be less than the cost to source the prototype materials commercially. However, we estimate the cost to produce at one and a half times the material cost to prototype or $30.69 per device with a significant financial investment required to build up the infrastructure necessary to produce, market, and sell the device. Each device would likely sell for $40 dollars with each sticker costing an additional $2.
Significance
The significance lies greatly with reactive measures rather than proactive measures. By being able to detect accidental tracheostomy decannulation faster than other metrics, such as oximeter alarm, health providers and caretakers can prevent serious damage due to oxygen deprivation.
Works Cited
Center for Devices and Radiological Health. "Overview of Device Regulation." U.S. Food and Drug Administration, FDA, 31 Aug. 2018, www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/overview-device-regulation.
"Medical Tube Safety Device." United States Patent: 9788583, 17 Oct. 2017, patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/srchnum.html&r=1&f=G&l=50&d=PALL&s1=9788583.PN.
Acknowledgements
-Dr. Nicholas Holekamp, CMO Ranken Jordan. Our primary contact at Ranken Jordan, Dr. Holekamp gave us a tour of the hospital, met with us to discuss his ideas for the project, and helped us establish metrics for success.
-Joseph Klaesner PhD, Professor of Biomedical Engineering at Washington University in St. Louis. Professor Klaesner assisted us with the project by guiding us on appropriate steps to take, and coordinated funding through the Biomedical Engineering Department at Washington University.
-The Biomedical Engineering Department in the McKelvey School of Engineering at Washington University in St. Louis for funding the project and guiding us through our undergraduate degree program.