Evaluating the effects of noninvasive spinal stimulation on gait parameters in cerebral palsy via markerless motion capture
Alexandra A. Portnova-Fahreeva1, Charlotte R. DeVol2, Victoria M. Landrum3, Siddhi R. Shrivastav1, Kristie F. Bjornson1, Chet T. Moritz1, Heather Feldner1, Katherine M. Steele1
1University of Washington, 2University of Florida, 3University of Michigan
INTRODUCTION
Cerebral palsy (CP) is the most common childhood motor disability [1]. CP often affects gait, requiring interventions to improve or support mobility. Recent research in noninvasive spinal stimulation has shown potential to improve motor control, spasticity, and gait in CP. A study that combined spinal stimulation and activity-based neurorehabilitation (2x/week for 8 weeks) demonstrated significant improvement in Gross Motor Function Measure scores for all 16 children with CP [2]. Additionally, 24-sessions of spinal stimulation with intensive treadmill training reduced spasticity for 4 children with CP [3]. While these early results suggest spinal stimulation may improve gait, changes in gait during stimulation remain largely unknown. Data on 9 children with CP suggesting a trend of increasing hip and knee excursion with stimulation on versus off [4].

Assessing the effects of stimulation on gait is especially important since stimulation parameters are often tuned while watching the child walk or perform other tasks. Monitoring gait during therapy may help optimize stimulation parameters, training intensity, and long-term effectiveness. An individualized approach is also critical in CP due to the diversity in impairments and movement patterns in this population. However, gait monitoring usually requires expensive and time-consuming motion capture, impractical in clinical settings. Markerless motion capture with consumer electronics can minimizes distraction for younger participants, decreases setup time, and potentially be used outside of the lab [5]. Here we evaluate the feasibility of markerless motion capture to quantify gait in children with CP with and without spinal stimulation.
METHODS
Participants. We recruited six children with CP, Gross Motor Functional Classification System Levels I-II (11±4.1 y/o, Table 1). Since spinal stimulation is believed to activate the spinal afferents, we excluded children who had botulinum toxin injections or other major surgical procedures in the prior six months or who had undergone selective dorsal rhizotomy.
Protocol. The participants walked on a treadmill for approximately 20 minutes in each condition: with stimulation ON and OFF. Treadmill speed was set based on the participant’s self-selected walking pace and, then kept the same for all conditions. Participants could use safety bars around the treadmill as needed for balance, and a physical therapist provided stand-by assist during walking both with and without spinal stimulation.
Spinal stimulation. We used an experimental SCONE spinal stimulator (SpineX) with stimulating electrodes applied to the skin over thoracic (T11) and lumbar (L1) dorsal spinous processes. Ground electrodes were placed on the anterior, superior iliac crests bilaterally. Stimulation intensity (Table 1) was selected by slowly increasing the stimulation in 5-10mA increments while monitoring the participant’s walking pattern and comfort. Biphasic rectangular pulses of 1ms duration were delivered at a frequency of 30Hz. Within these pulses, a 10kHz carrier frequency was delivered to reduce sensations as the current crosses the skin, enabling higher stimulation intensities compared to other noninvasive approaches [6]. No painful sensations were reported by any of the participants during the stimulation.
|
Age |
Sex |
GMFCS |
Intensity |
Speed (m/s) |
Excluded Gait Cycles (%) Stim OFF Stim OFF |
||
|
S1 |
15 |
M |
II |
35/25 |
0.90 |
22.3±3.6 |
20.3±16.4 |
|
S2 |
6 |
F |
II |
10/20 |
0.60 |
23.0±1.8 |
23.0±3.6 |
|
S3 |
6 |
F |
II |
10/15-20 |
0.65 |
21.4±5.0 |
13.4±6.8 |
|
S4 |
13 |
F |
I |
20/30 |
1.00 |
10.7±3.5 |
12.8±2.1 |
|
S5 |
15 |
M |
II |
30/20 |
1.05 |
16.3±2.0 |
11.5±1.5 |
|
S6 |
11 |
M |
II |
10/20 |
0.80 |
14.6±7.4 |
11.5±4.1 |
Motion Capture. For biomechanical analyses, we used OpenCap, an open-source platform that facilitates markerless motion capture using two iPad/iPhone cameras [5]. Developed for biomechanics research and human movement analysis, OpenCap bypasses the need for complex, traditional marker-based motion capture systems. One-minute long OpenCap videos were recorded at 60 frames/second approximately every five minutes (Fig. 1, top left). Since OpenCap tracks the largest person in the frame, custom code was added enabling users to select which individual to track in each camera to account for a physical therapist or researcher in frame with a participant.
Kinematic Analysis. Kinematic analyses were performed via OpenCap to estimate hip flexion/extension for both legs during gait. We chose to focus on this joint as it plays an important role in gait for children with CP, drives step length, and has been noted by our clinicians and prior research as a kinematic feature that can be observed to tune spinal stimulation intensity [4]. Prior research has also demonstrated that OpenCap has good accuracy for the hip relative to marker-based systems, but can have greater variability for the ankle and knee joints [7]. Since children with CP utilize altered gait patterns, we used the marker locations estimated from OpenCap with the Lai-Arnold Modified model [8] in OpenSim to perform inverse kinematics (Fig. 1, top right). Because OpenCap actively relies on machine learning models and can exhibit unexpected behavior, gait cycles during which the kinematics had high deviations were determined using a gait outlier detection method [9] and excluded from analysis. The cutoff value used for outlier detection was set to 2.0, which was equivalent to 2 standard deviations.
Prior research has shown increased inter-trial variability for markerless systems when evaluating gait kinematics [10]. For these reasons, it is suggested to average kinematic signals over gait cycles. As a result, we evaluated the peak hip flexion/extension angles for an average hip angle across all gait cycles in a single one-minute trial for each participant. We also evaluated inter-leg symmetries, which prior research has suggested may change with stimulation and is observed by clinicians [4]. To quantify symmetry, right hip to left hip angles were shifted to align the peak hip extensions of both legs. Correlation coefficients were calculated between each cycle trajectory and the mean trajectory for the 1-minute trial as described in Gad et al [4]. Mean correlation coefficients for the hip joints were then calculated and plotted for each one-minute trial. A value of 1 indicates perfect symmetry.
A Shapiro-Wilk test was used to evaluate normality of peak angle data, revealing the distributions were not-normal. A non-parametric, two-sample Kolmogorov Smirnov test was used to compare the differences within each participant for stimulation ON versus OFF conditions.
RESULTS
The effect of spinal stimulation on hip angles measured via OpenCap across 20-minute training sessions varied across participants (Fig. 2). Notably, characteristic of children with CP, the participants in this study had different gait patterns – some with excessive hip flexion and others with limited hip extension. Overall, hip range of motion was similar with stimulation ON and OFF for most participants, although S3 showed significant increase in hip flexion and decrease in hip extension for the left leg (p=0.04 and p=0.004, respectively) with stimulation ON. A significant decrease in right hip flexion was also observed in S1 and S2 (p=0.04 and p=0.01, respectively).
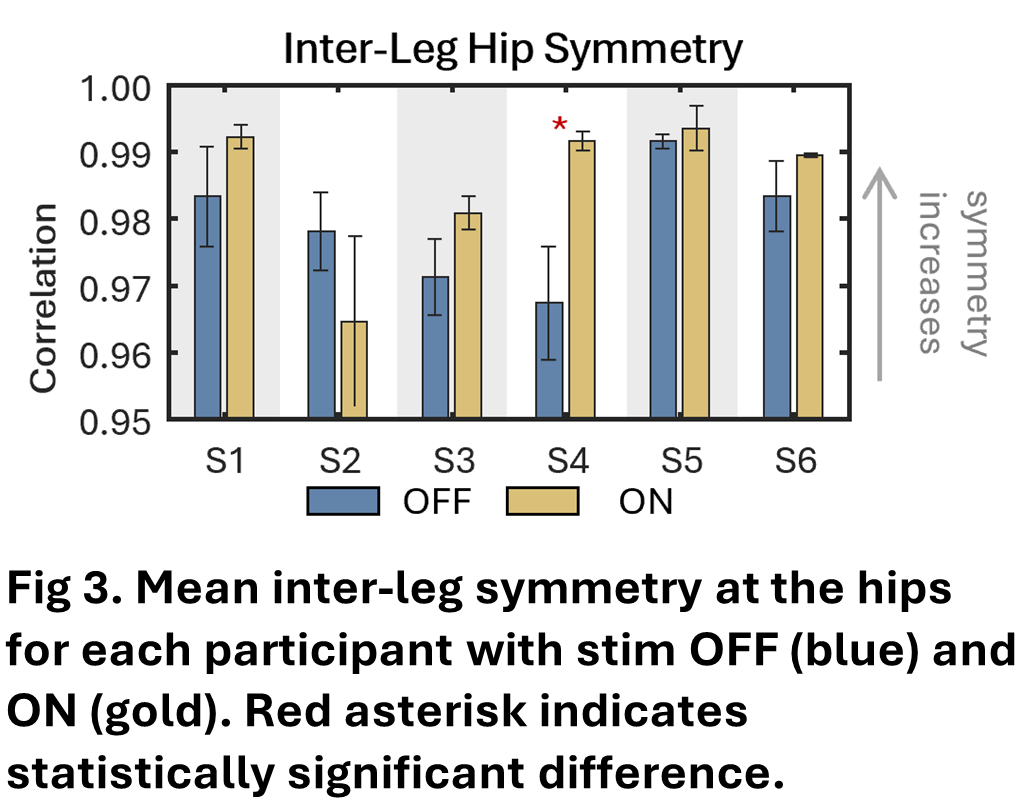
Hip correlation coefficients were relatively high for inter-leg symmetry parameters (0.95-1.00, Fig. 3). Only S4 had a significant increase in symmetry with simulation ON (p=0.04), although there was a trend toward increasing symmetry with stimulation for 5 of the 6 participants.
While prior research has reported good accuracy for the hip with OpenCap, we found that 8.2-31.9% of gait cycles were excluded for the chosen cutoff value due to inconsistencies in the markerless tracking (Table 1).

(* indicates p≤0.05, ** indicates p≤0.01).
However, the OpenCap set-up was deemed as feasible by the clinical research team based on the minimal time and resources required for set-up during testing sessions.
DISCUSSION
We used OpenCap to evaluate the feasibility of markerless motion capture to capture the effects of noninvasive spinal stimulation on gait parameters in children with CP. In past research, OpenCap has shown kinematic errors of 5.4 degrees for hip flexion during walking in unimpaired adults when compared to marker-based systems; however, higher errors have been reported for hip flexion angle for clinical pathological gait patterns [11]. This may be due to the fact that OpenCap has been largely trained on unimpaired, adult populations. Additionally, higher inter-trial variability has been reported for markerless motion capture [10]. Similar results can be observed in our study as a large number of gait cycles had to be excluded from analysis due to inconsistencies in markerless tracking.
Patient-specific differences in hip kinematics were observed with stimulation ON versus OFF, which could be used to guide the selection and optimization of stimulation parameters and gait training. Although no consistent trends were observed across all participants, the variability between participants is characteristic of this population. This work highlights the need for individualized approaches for monitoring the impact of interventions on kinematics. The participants in our study had total hip excursion angles that were greater than the average 30 degrees reported by Gad and colleagues (2021). These differences may also be due to how hip angles are measured with OpenCap versus traditional marker-based systems. Coefficients of correlation in Gad et al. were 0.9-1.0, in a similar range as found in our study, despite the fact that participants in the prior study were more severely involved. Notably, the participants in our study had no prior experience walking with spinal stimulation, which may have contributed to the observed variability. Additionally, participants were all GMFCS Level I-II, unlike those in Gad et al., where more immediate effects were observed in children with higher GMFCS levels.
Markerless motion capture provides a tool to monitor and observe changes in gait during rehabilitation when the time and resource burden of traditional motion capture systems are not feasible. Our analysis revealed the overall stability of the system for monitoring joint angles, although a portion of gait cycles had to be excluded due to inconsistencies in tracking. However, as seen in the bottom of Fig. 1, joint angles were consistent across retained gait cycles. Further, since many gait cycles could be captured during 1-minute bouts with OpenCap, this system allowed for in-session tracking during training despite excluding a relatively large portion of gait cycles. It is also important to consider that the definition of joint angles may differ from traditional marker-based systems. In particular, pelvic and transverse plane positions are challenging for markerless systems to estimate [5], which may have contributed to larger peak hip flexion and extension values in this study. However, these differences are expected to be consistent across trials for a given participant, such that markerless systems can be useful for quantifying participant-specific changes across time or interventions. This study was also limited by the number of participants and short time to test and refine spinal stimulation parameters. Future studies systematically investigating gait changes with varying spinal stimulation intensities and comparing results to traditional marker-based motion analysis are crucial for optimizing novel interventions to improve gait in CP.
CONCLUSIONS
This study highlights the potential of using markerless motion capture to monitor and analyze gait biomechanics in children with CP. Our findings revealed patient-specific variations in hip kinematics with spinal stimulation, underscoring the importance of individualized approaches to optimize treatment parameters. Despite limitations, our study supports future research aimed at refining spinal stimulation protocols and evaluating the effectiveness of markerless motion capture in clinical settings. Such advancements will be key in optimizing interventions to improve gait and function in children with CP.
REFERENCES
[1] Yeargin-Allsopp, M., et al. (2008). Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics, 121(3), 547–554.
[2] Hastings, S., et al. (2022). A pilot study combining noninvasive spinal neuromodulation and activity-based neurorehabilitation therapy in children with cerebral palsy. Nature Communications, 13(1), 5660.
[3] Shrivastav, S. R., et al. (2023). Transcutaneous Spinal Stimulation and Short-burst Interval Treadmill Training Improve Spasticity and Walking Function in Children with Cerebral Palsy. medRxiv.
[4] Gad, P., et al. (2021). Transcutaneous Spinal Neuromodulation Reorganizes Neural Networks in Patients with Cerebral Palsy. Neurotherapeutics, 18(3), 1953–1962.
[5] Uhlrich, S. D., et al. (2023). OpenCap: Human movement dynamics from smartphone videos. PLoS Computational Biology, 19(10), e1011462.
[6] Inanici, F., et al. (2018). Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE TNSRE, 26(6), 1272–1278.
[7] Turner, J. A., et al. (2024). Validation of OpenCap: A low-cost markerless motion capture system for lower-extremity kinematics during return-to-sport tasks. Journal of Biomechanics, 112200.
[8] Lai, A. K., et al. (2017). Why are antagonist muscles co-activated in my simulation? A musculoskeletal model for analysing human locomotor tasks. Annals of Biomedical Engineering, 45, 2762–2774.
[9] Sangeux, M., & Polak, J. (2015). A simple method to choose the most representative stride and detect outliers. Gait & Posture, 41(2), 726-730.
[10] Horsak, Brian, et al. (2024) Inter-trial variability is higher in 3D markerless compared to marker-based motion capture: Implications for data post-processing and analysis. Journal of Biomechanics, 166, 112049.
[11] Horsak, Brian, et al. (2023). Concurrent validity of smartphone-based markerless motion capturing to quantify lower-limb joint kinematics in healthy and pathological gait. Journal of Biomechanics, 159, 111801.