Baseline Predictors of Improvements in Clinical Balance and Gait Outcomes After Treadmill Oscillation Walking Training in Individuals Post-Stroke
Wenxi Zhang1, Jason Tsai1, Keng-Hung Shen M.D., Ph.D.1, Hao-Yuan Hsiao Ph.D.1
1Department of Kinesiology and Health Education, University of Texas at Austin
INTRODUCTION
Stroke remains a leading cause of long-term disability worldwide, often resulting in persistent impairments in walking and balance that limit independence and reduce quality of life [1], [2]. While many rehabilitation interventions have demonstrated efficacy in clinical trials, interpreting treatment effects can be difficult due to individual variability in impairment severity and the sensitivity of outcome measures [3]. Some individuals may experience substantial benefits, whereas others show little to no improvement [4]. Identifying predictors of treatment response is therefore critical to tailoring rehabilitation and optimizing outcomes.
Regression analysis offers a robust method for examining heterogeneity in treatment effects [5], [6]. Recent studies suggest that baseline characteristics such as age, balance ability, and lower-limb strength may influence post-stroke rehabilitation outcomes [7], [8], [9].
The present study investigates whether baseline demographic, clinical, and biomechanical variables predict improvements following six weeks of Treadmill Oscillation Walking (TOW)—a novel gait intervention involving medial-lateral oscillations to challenge dynamic balance. In a preliminary study, most participants showed gains in the Berg Balance Scale (BBS), Dynamic Gait Index (DGI), self-selected walking speed (SSWS), and fastest walking speed (FWS), though the magnitude of improvement varied. The primary objective was to identify predictors of clinical improvement following TOW. A secondary aim was to explore whether changes in hip and knee joint torque were associated with clinical outcome gains.
METHODS
Seventeen individuals with chronic stroke were recruited from St. David’s Rehabilitation Hospital. Two withdrew, leaving 15 who completed the intervention (mean age = 59.05 years, SD = 11.88; 8 males; mean time since stroke onset = 4.84 years, SD = 4.82; 6 with left-side hemiparesis). The study was approved by the Institutional Review Board at The University of Texas at Austin.
Treadmill Oscillation Walking (TOW) Protocol
Participants completed 18 training sessions over six weeks (three sessions per week). Each session involved six 6-minute bouts of treadmill walking at self-selected speed on a split-belt treadmill (Motek Inc., Columbus, OH). After steady-state walking was achieved, the treadmill applied 1 cm amplitude sinusoidal medial-lateral oscillations at a frequency matched to each participant’s baseline cadence using D-Flow software. The oscillation frequency increased weekly by 5% to promote adaptation [10]. Participants wore a safety harness and could use handrails or ankle-foot orthoses (AFOs) if typically required. Pre- and post-intervention assessments were conducted within one week of training.
Clinical Assessments
Clinical outcomes were assessed by a licensed physical therapist. Static balance was measured with the BBS [11]; dynamic balance during gait was assessed using the DGI [12]. SSWS and FWS were determined from the middle 6 meters of a 10-meter walk [13] and walking endurance was measured using the 6-minute walk test (6MWT). Assistive devices were permitted during testing.
Lower-Limb Joint Torque Assessment
Isometric strength of the hip abductors and knee extensors was assessed bilaterally using a Humac Norm dynamometer (Computer Sports Medicine, Inc., Stoughton, MA). Participants were seated upright with hips flexed to 90°, trunk inclined at 85°, and strapped securely. Knee extension torque was tested with the knee at 60° flexion and resistance applied just above the ankle. Hip abduction torque was tested at 15° of abduction with resistance applied laterally to the thigh. Two warm-up trials preceded two maximal effort trials for each joint, separated by 60 seconds of rest. Peak torque values were recorded and normalized to body weight.
Rate of Force Development (RFD)
RFD was calculated from the trial with the highest torque. Onset was defined as the point where torque exceeded 2.5% of peak, and the slope from onset to 200 ms was computed [14]. All torque and RFD values were normalized to body weight.
STATISTICAL ANALYSES
Stepwise linear regression identified predictors of changes in clinical outcomes (ΔBBS, ΔDGI, Δ6MW, ΔFWS, ΔSSWS). Baseline predictors included age, sex, initial clinical scores, and bilateral hip and knee torque and RFD. Secondary analyses examined whether post-intervention changes in torque and RFD predicted clinical improvements. Significance was set at P < .05, with analyses conducted in R and SPSS.
RESULTS
Baseline Predictors of Clinical Gait and Balance Outcomes
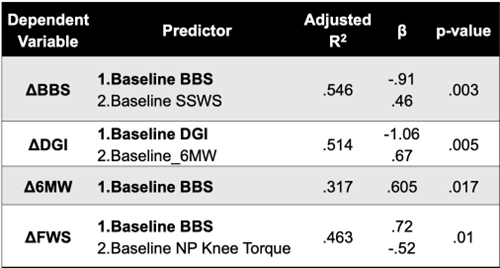
Table 1 presents the results of the stepwise linear regression analyses of baseline predictors. Baseline BBS, Baseline SSWS were predictors for the change of the BBS (adjusted R2 = 0.546, P < 0.05). Baseline DGI and Baseline 6MW were predictors for the change of DGI (adjusted R2 = 0.514, P < 0.05). Baseline BBS was predictor for the change of 6MW (adjusted R2 = 0.317, P < 0.05). Baseline BBS and Baseline non-paretic knee extensor were predictors for the change of FWS (adjusted R2 = 0.463, P < 0.05). No predictor was detected for changes in SSWS. Age, sex, and baseline hip joint torques, and RFDs were not predictors for changes in any of the clinical outcomes.
Relationships Between Changes in Strength Measurements and Changes in Clinical Outcomes

Table 2 presents the results of the stepwise linear regression analyses for the predictive effect of changes in muscle strength measurements. Changes in paretic hip abductor torque was the only predictor of changes in the 6MW (adjusted R2 = 0.280, P < 0.05) and SSWS (adjusted R2 = 0.253, P < 0.05) following TOW intervention. Changes in knee extensor strength on both the paretic and non-paretic sides, as well as changes in RFD for both joints and sides, did not predict changes in any of the clinical outcomes.
DISCUSSION
We determined the baseline predictors of clinical gait and balance outcomes for individuals post-stroke following six weeks of TOW training. Improvements in strength and RFD of the paretic limb were associated with enhanced walking speed, endurance, and balance. These findings highlight the importance of targeted strength training in stroke rehabilitation.
Our findings, that baseline BBS and DGI positively contributed to the prediction of ΔSSWS and Δ6MW, are consistent with a previous study that reported that participants with lower BBS scores were less likely to transition from lower to higher walking speed categories [3]. Additionally, other studies have indicated that individuals with a BBS score below 20 and a Functional Independence Measure (FIM) walk score of 1 or 2 at admission are likely to achieve only household ambulation (<0.4 m/s) at discharge [16]. These findings highlight that balance control ability is likely a fundamental prerequisite for improving walking speed.
We found negative relationships between baseline and intervention-induced balance measures (i.e. BBS and DGI). That is, individuals with poor balance performance at baseline showed greater treatment gains in balance after TOW training. A potential explanation is that unlike walking speed, BBS and DGI assessments have maximum scores. Thus, participants with high baseline scores may have limited range for improvement with this ceiling effect [17]. Taken together, these results suggested that the TOW training program induces more functional balance gains in individuals with lower baseline balance abilities, while improving walking speed in those who have better balance ability.
Age has been reported as a predictor of recovery in ambulation and independent gait [18], [19]. It has also been identified as a predictor of improvements in walking speed two months post-stroke [3]. However, age did not influence responses to the TOW intervention, potentially because all our participants were in the chronic stage with limited self-recovery.A previous study using a machine learning approach to develop a prediction model for inpatient stroke rehabilitation found that clinical test scores at admission were the most important predictors of outcomes at discharge [20]. Our results expand their findings by showing that baseline assessments of balance and gait can predict gait intervention outcomes in persons with chronic stroke. This information could help clinicians develop personalized treatment plans that optimizes functional recovery gains for stroke survivors.
Previous studies have emphasized the importance of hip abduction in gait performance. [21] found that greater accuracy in reaching the peak hip abduction angle during a hip oscillation task, a controlled assessment of active hip abduction and adduction, was associated with improved gait performance, reflected in wider paretic step widths and longer paretic step durations in individuals post-stroke. Similarly, [22] found that individuals with chronic stroke who showed better lateral weight transfer, as indicated by greater hip abductor moment and reduced lateral placement of the paretic foot relative to the center of mass, had faster comfortable walking speeds. In the present study, TOW was delivered in the medial-lateral direction. Because hip ab/adduction torque is a key mechanism of maintaining lateral balance stability [23], TOW likely further engaged the hip ab/adductor muscles during walking. As such, participants who gained greater hip abductor strength on the paretic side also increased faster walking speed. These results underscore the pivotal role of the paretic limb in achieving successful rehabilitation outcomes. Moreover, prior work by [19] demonstrated that impairments in voluntary lower limb control, such as abnormal hip extension-abduction coupling after stroke, can further hinder paretic limb function. Thus, an approach that targets synergetic lower limb joint torque production is likely useful for gait function recovery post-stroke. In this regard, TOW holds potential for further development as a tool that concurrently engages hip ab/adduction with extension during ongoing gait to advance post-stroke gait rehabilitation.
CONCLUSION
This study shows that individuals post stroke with different initial balance abilities could benefit from TOW training in various ways. Participants with better balance control ability are likely to improve their walking speed after TOW intervention. In contrast, those with lower baseline balance scores may show more improvements in balance outcomes following TOW. Baseline screening for balance function (i.e. BBS) may enhance post-stroke gait intervention effectiveness and optimize clinical resource allocation.
REFERENCES
[1] M. Grau-Pellicer, A. Chamarro-Lusar, J. Medina-Casanovas, and B.-C. Serdà Ferrer, “Walking speed as a predictor of community mobility and quality of life after stroke,” Topics in Stroke Rehabilitation, vol. 26, no. 5, pp. 349–358, Jul. 2019, doi: 10.1080/10749357.2019.1605751.
[2] P. Langhorne, J. Bernhardt, and G. Kwakkel, “Stroke rehabilitation,” The Lancet, vol. 377, no. 9778, pp. 1693–1702, May 2011, doi: 10.1016/S0140-6736(11)60325-5.
[3] B. H. Dobkin et al., “Prediction of responders for outcome measures of Locomotor Experience Applied Post Stroke trial,” J Rehabil Res Dev, vol. 51, no. 1, pp. 39–50, 2014, doi: 10.1682/JRRD.2013.04.0080.
[4] G. Morone et al., “Who May Benefit From Robotic-Assisted Gait Training?: A Randomized Clinical Trial in Patients With Subacute Stroke,” Neurorehabil Neural Repair, vol. 25, no. 7, pp. 636–644, Sep. 2011, doi: 10.1177/1545968311401034.
[5] D. M. Kent, P. M. Rothwell, J. P. Ioannidis, D. G. Altman, and R. A. Hayward, “Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal,” Trials, vol. 11, no. 1, p. 85, Dec. 2010, doi: 10.1186/1745-6215-11-85.
[6] X. Sun et al., “Subgroup Analysis of Trials Is Rarely Easy (SATIRE): a study protocol for a systematic review to characterize the analysis, reporting, and claim of subgroup effects in randomized trials,” Trials, vol. 10, no. 1, p. 101, Dec. 2009, doi: 10.1186/1745-6215-10-101.
[7] E. Burke, B. H. Dobkin, E. A. Noser, L. A. Enney, and S. C. Cramer, “Predictors and Biomarkers of Treatment Gains in a Clinical Stroke Trial Targeting the Lower Extremity,” Stroke, vol. 45, no. 8, pp. 2379–2384, Aug. 2014, doi: 10.1161/STROKEAHA.114.005436.
[8] H. Hsiao, J. S. Higginson, and S. A. Binder-Macleod, “Baseline predictors of treatment gains in peak propulsive force in individuals poststroke,” J NeuroEngineering Rehabil, vol. 13, no. 1, p. 2, Dec. 2016, doi: 10.1186/s12984-016-0113-1.
[9] C. R. Taylor, “Force development during sustained locomotion: a determinant of gait, speed and metabolic power,” J Exp Biol, vol. 115, pp. 253–262, Mar. 1985, doi: 10.1242/jeb.115.1.253.
[10] M. Pohl, J. Mehrholz, C. Ritschel, and S. Rückriem, “Speed-Dependent Treadmill Training in Ambulatory Hemiparetic Stroke Patients: A Randomized Controlled Trial,” Stroke, vol. 33, no. 2, pp. 553–558, Feb. 2002, doi: 10.1161/hs0202.102365.
[11] M. Godi, F. Franchignoni, M. Caligari, A. Giordano, A. M. Turcato, and A. Nardone, “Comparison of Reliability, Validity, and Responsiveness of the Mini-BESTest and Berg Balance Scale in Patients With Balance Disorders,” Physical Therapy, vol. 93, no. 2, pp. 158–167, Feb. 2013, doi: 10.2522/ptj.20120171.
[12] S. Batool, H. Zafar, S. A. Gilani, A. Ahmad, and A. Hanif, “Intrarater and interrater reliability of the dynamic gait index in post stroke patients with eye movement disorders,” Journal of Bodywork and Movement Therapies, vol. 35, pp. 38–42, Jul. 2023, doi: 10.1016/j.jbmt.2023.04.015.
[13] H. Busk, P. Holm, S. T. Skou, S. Seitner, T. Siemsen, and T. Wienecke, “Inter-rater reliability and agreement of 6 Minute Walk Test and 10 Meter Walk Test at comfortable walk speed in patients with acute stroke,” Physiotherapy Theory and Practice, vol. 39, no. 5, pp. 1024–1032, May 2023, doi: 10.1080/09593985.2022.2030830.
[14] P. Aagaard, E. B. Simonsen, J. L. Andersen, P. Magnusson, and P. Dyhre-Poulsen, “Increased rate of force development and neural drive of human skeletal muscle following resistance training,” Journal of Applied Physiology, vol. 93, no. 4, pp. 1318–1326, Oct. 2002, doi: 10.1152/japplphysiol.00283.2002.
[15] K. Takeda et al., “Relationship between the rate of force development in knee extensor muscles and gait speed in patients with chronic stroke: A cross-sectional study,” NRE, vol. 43, no. 4, pp. 425–430, Jan. 2019, doi: 10.3233/NRE-182455.
[16] M. D. Bland et al., “Prediction of Discharge Walking Ability From Initial Assessment in a Stroke Inpatient Rehabilitation Facility Population,” Archives of Physical Medicine and Rehabilitation, vol. 93, no. 8, pp. 1441–1447, Aug. 2012, doi: 10.1016/j.apmr.2012.02.029.
[17] A. Zupanc and U. Puh, “Validity, responsiveness, floor and ceiling effects of the Berg Balance Scale in patients with Guillain-Barré syndrome,” International Journal of Rehabilitation Research, vol. 44, no. 4, pp. 364–369, Dec. 2021, doi: 10.1097/MRR.0000000000000499.
[18] L. K. Kwah, L. A. Harvey, J. Diong, and R. D. Herbert, “Models containing age and NIHSS predict recovery of ambulation and upper limb function six months after stroke: an observational study,” Journal of Physiotherapy, vol. 59, no. 3, pp. 189–197, Sep. 2013, doi: 10.1016/S1836-9553(13)70183-8.
[19] I. Sánchez-Blanco, C. Ochoa-Sangrador, L. López-Munaín, M. Izquierdo-Sánchez, and J. Fermoso-García, “Predictive model of functional independence in stroke patients admitted to a rehabilitation programme,” Clin Rehabil, vol. 13, no. 6, pp. 464–475, Dec. 1999, doi: 10.1191/026921599672994947.
[20] Y. Harari, M. K. O’Brien, R. L. Lieber, and A. Jayaraman, “Inpatient stroke rehabilitation: prediction of clinical outcomes using a machine-learning approach,” J NeuroEngineering Rehabil, vol. 17, no. 1, p. 71, Dec. 2020, doi: 10.1186/s12984-020-00704-3.
[21] J. C. Dean, A. E. Embry, K. H. Stimpson, L. A. Perry, and S. A. Kautz, “Effects of hip abduction and adduction accuracy on post-stroke gait,” Clinical Biomechanics, vol. 44, pp. 14–20, May 2017, doi: 10.1016/j.clinbiomech.2017.02.013.
[22] H. Hsiao, V. L. Gray, R. A. Creath, S. A. Binder-Macleod, and M. W. Rogers, “Control of lateral weight transfer is associated with walking speed in individuals post-stroke,” Journal of Biomechanics, vol. 60, pp. 72–78, Jul. 2017, doi: 10.1016/j.jbiomech.2017.06.021.
[23] W. Jeon, J. Borrelli, and H.-Y. Hsiao, “Effects of Visual Input Absence on Balance Recovery Responses to Lateral Standing Surface Perturbations in Older and Younger Adults,” Journal of Applied Biomechanics, vol. 39, no. 3, pp. 184–192, Jun. 2023, doi: 10.1123/jab.2022-0029.