Kati P. Liegl, Kathy L. Rust, Roger O. Smith
Rehabilitation Research Design & Disability (R2D2) Center,University of Wisconsin-Milwaukee
Abstract
This project purpose was to introduce a new rehabilitation tool and the associated rehabilitation intervention (Cranial Nerve Non-Invasive Neuromodulation [CN-NINM]) on functional gait and balance for people with traumatic brain injuries. The intervention has been tested on various disability groups and is being evaluated around the world. This needs assessment and systematic study highlights the need to document withdrawal effects in addition to reporting benefits. While using the CN-NINM intervention, each participant’s scores are anticipated to significantly improve for qualitative and quantitative measures. During withdrawal periods each participant’s scores are expected to significantly decrease based on previous studies. Scores during the second withdrawal period are expected to stay slightly higher than the first withdrawal period. This study will add to available research on the new intervention device and technique which may have significant effects on rehabilitation services.
Background
Theoretical Background
Neurorehabilitation utilizes neuroplasticity of the brain to restructure and relearn information, typically after a neurotrauma. Individuals most commonly treated are those who have had a cerebral vascular incident, traumatic brain injury or spinal cord injury. Neurorehabilitation therapists use multiple techniques to help the patient improve, either by helping the patient relearn and improve previous skills, compensate with alternative techniques, or providing assistive technologies that may be temporary or permanently used. A related field, neuromodulation, alters the nervous system with the use of electricity or medications. Current, commonly used neuromodulation interventions include baclofen pumps, spinal cord stimulators, deep brain stimulators and transcranial magnetic stimulation. These interventions are not typically thought to have long lasting effects (Kern & Kumar, 2007; O’Malley, Ro, & Levin, 2006), however, the effects noted during and directly after the application of the medication or electricity have substantial effects.
In the 1960’s, scientists explored the ability to use sensory substitution. Bach-y-Rita, a primary founding scientist, used electrical stimulation as a means of providing sensory information to a participant in an alternative format. This research focused primarily on providing visual information to a participant using electrical stimulation rather than relying on sight. Participants included individuals with vision impairments as well as with occluded vision. The electrical stimulation that provided the visual information was applied various places on the body, including the fingers, abdomen, back, forehead, and tongue. The tongue had the most favorable results for several reasons. The mouth provides a secure, discreet, and isolated environment for the stimulation, maintains a relatively constant temperature and is not typically affected by environmental stimuli such as a breeze. Saliva maintains a constant pH+ and reduces the stimulation required for perception and interpretation. The tongue, because of the location and density of nerve receptors, requires less stimuli to respond than elsewhere on the body, including the fingers. In addition, participants have not indicated the sensation of the stimulation is unpleasant or painful (Bach-y-Rita & Kercel, 2003)
During the studies completed on sensory substitution, researchers and participants noted other changes in function, not related to the sensory substitution. These changes, observed in many of the participants receiving electrical stimulation on the tongue, led to another line of research to study the effects of the stimulation when used for purposes in addition to the sensory substitution. The first device that came from this line of research, the BrainPort™, used electrical stimulation of the tongue to provide biofeedback for balance. Through the observations noted in the initial sensory substitution and the BrainPort™, another line of research was initiated to determine the effects of the electrical stimulation when not used to provide environmental information. This intervention was called Cranial Nerve Non-Invasive Neuromodulation (CN-NINM).
The Rehabilitation Technologies
A novel neuromodulation technique, CN-NINM, was created at the University of Wisconsin-Madison in the Tactile Communications & Neurorehabilitation Laboratory (TCNL). This intervention has two primary components: 1) the use of the Portable Neuromodulation Stimulator (PoNS™) device to deliver small, safe doses of electric current to the tongue, and 2) targeted training activities that are personalized for each participant based on presenting symptoms and typically performed concurrently with the stimulation. The targeted maximal challenge training activities are similar to activities performed during standard neurorehabilitation but are additionally maintained for 20 minutes each to maximize neuroplastic effect. Previous research using the PoNS™ device and CN-NINM intervention have addressed impairments in balance and gait for several disability categories including traumatic brain injury, Parkinson’s disease, and stroke, among others. Currently, all completed studies have examined the effects of the CN-NINM intervention on balance and gait, and used balance and gait targeted training activities (Danilov, Tyler, Rust, Kaczmarek, & Subbotin, n.d.; Tactile Communications & Neurorehabilitation Laboratory, 2011; Wildenberg, Tyler, Danilov, Kaczmarek, & Meyerand, 2010; Wildenberg, Tyler, Danilov, Kaczmarek, & Meyerand, 2011).
PoNS™ Device
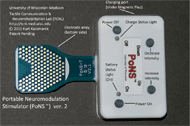 Figure 1: PoNS™ device d
Figure 1: PoNS™ device dThe PoNS™ device has been created with conscious thought given to usability and safety. The case for the PoNS™ device is approximately 68 mm wide by 45 mm long by 15 mm thick and is approximately 56 grams. The oral tab on the T-shaped device that provides the stimulation contains 143 electrodes and fits on the anterior portion of the tongue, held lightly in place by the lips. The PoNS™ device uses an unbalanced biphasic waveform designed to ensure net zero current (Kaczmarek, 2011) to reduce the chance of tissue irritation and has 19 V max and 6mA operational limits. Pulses are delivered to the tongue in triplets of pulses at 5 ms intervals every 20 ms. The subject can control the pulse-width (0.4-0.6 µs) by adjusting the intensity buttons on the device. The buttons on the device allow the stimulation to be turned on and off and increased or decreased in intensity. Each time the device is turned off, the intensity resets to the lowest level, requiring the subject to adjust it to a comfortable level each time (Kaczmarek, 2011).
The PoNS™ device uses a rechargeable lithium-ion battery. The device cannot be in use while charging, thereby preventing the risk of electrical shock. The tab, except for the electrodes, is covered with an FDA approved USP Class VI biocompatible polymer to prevent saliva from harming the electronics. The device is cleaned with isopropyl alcohol after use.
CN-NINM TBI withdrawal StudY
The study design described here is the first systematic effort to document the withdrawal effects from using the CN-NINM intervention as well as the benefits during use for balance and gait. This step is critical for obtaining FDA approval and integral in understanding appropriate uses for the device. The CN-NINM intervention and PoNS™ device are intended to be used as a rehabilitation tool, therefore understanding it is meant to be combined with the skilled expertise of a therapist. This study design adds to the clinically relevant intervention information. This study has received approval from the Institutional Review Board at the University of Wisconsin-Milwaukee.
Participants
Four participants will complete the present study and are recruited through local traumatic brain injury support and education groups. All participants are individuals who previously had a traumatic brain injury that resulted in a balance or gait impairment. Participants cannot be receiving any rehabilitation and cannot live in a facility that provides assistance (such as assisted living, group home, or skilled nursing facility). They must be between 18 and 65 years old and be their own legal guardian.
Methods
The study used a structured, four person, single subject experimental design. Each participant completes an A-B-A-B-A design where ‘A’ indicates a baseline or withdrawal phase, and ‘B’ indicates the CN-NINM intervention phase. Each phase lasts one week (Monday-Friday, with no data collection completed on weekends).
The independent variable is the CN-NINM intervention and the two primary dependent variables are 1) quality of functional gait and balance tasks, as measured by the Community Balance & Mobility Scale, and 2) confidence completing common balance and gait tasks, as measured by a modified version of the Gait Efficacy Scale. Data collection for the Community Balance & Mobility Scale is completed by a trained therapist and researcher blinded to the intentions of the study.
Protocol
Participants come to the laboratory every day (Monday –Friday) for five concurrent weeks and one additional day prior to beginning the five weeks. On the first day, participants complete the assessments used during the study. This data is thrown out and used to eliminate a learning curve due to familiarity with the assessments during the first week. The first, third and fifth weeks each are completed the same. Each day during these weeks, participants are evaluated on the Community Balance and Mobility (CB&M) Scale. On Friday, participants independently complete the following three self-assessment surveys: the modified Gait Efficacy Scale (GES-m), the Participation Objective Participation Subjective (POPS), and the Community Integration Questionnaire (CIQ).
The second and fourth weeks are intervention weeks and completed the same way. On the first day of the second week, participants receive the PoNS™ device and are instructed in proper wear, care, and use of the device. Each day during the second and fourth weeks, participants come to the laboratory twice per day to complete the intervention. Each time per day, participants complete the same interventions, however, prior to beginning the intervention in the afternoon, participants are evaluated on their performance on the CB&M Scale, and on both Fridays participants complete the GES-m, POPS, and CIQ. Each morning and afternoon intervention session consist of three primary components; participants complete three 20-minute training sessions for balance, relaxation, and gait. During the balance and gait targeted training sessions, the PoNS™ device is used to provide neuromodulation during the trainings. The PoNS™ device is not worn during the relaxation training. Each training session consists of individualized training tasks specific to the subject’s impairments.
Typical balance tasks include standing with his/her eyes closed, with or without shoes, on the floor or foam. Participants attempt to work up to standing in tandem on foam with their eyes closed, however, the intensity of the training depends on the functional level of the participant. Typical gait training tasks include walking over ground or on a treadmill, both forward and backward. Primary targets for gait training are to obtain equal stride lengths, toe clearance, upright posture, and appropriate arm swing. Relaxation trainings focus on increased body awareness and awareness of breathing. The PoNS™ device is not worn during the relaxation trainings.
Data Analysis
Several well-established single subject data analyses methods are utilized because of the exploratory nature of the data and lack of previous research on the withdrawal effects. Data is analyzed visually by the primary researcher as well as an expert in single subject design data analysis to strengthen findings (Ottenbacher, 1986). Visual analyses examine the level, trend, variability, immediacy of effect, overlap and consistency of data patterns across similar phases, per the Kratochwill et al. (2010) guidelines. Data will be examined several ways to ensure proper fit of analyses. Basic trend lines (Solanas, Manolov, & Onghena, 2010), percentage of data overlap (Parker, Vannest, &Davis, 2011), and two standard deviation band method approaches (Nourbakhsh & Ottenbacher, 1994) are also used. Data analysis methods are designed to describe trends noted between phases for each participant as well as describe trends noted between participants.
Expected Outcomes
Based on prior research, participant function on balance and gait tasks is expected to significantly improve during each intervention week. Similarly, it is expected that each participants’ confidence completing balance and gait tasks will increase significantly during intervention weeks. Previous observational reports noted that participant function decreased when not completing the intervention, therefore it is hypothesized that participant performance on the functional mobility and balance scale will significantly decrease during each of the withdrawal weeks. It is expected that although function will decrease during each withdrawal week, the resulting level of function will be slightly higher during the second withdrawal week than during the first withdrawal phase. Participant confidence with balance and gait tasks is expected to follow the same trends as functional abilities.
Conclusion
The design presented for this study is critical for several reasons. Although an exploratory single subject design, the study design provides significant findings to facilitate improved structure on more rigorous research to be completed in the future. In addition, this study provides the first documented withdrawal effects. This information is critical to future research structure, FDA approval, and rehabilitation implementation.
References
Bach-y-Rita, P., & Kercel, S. W. (2003). Sensory substitution and the human-machine interface. Trends in Cognitive Sciences, 7(12), 541-546. doi: 10.1016/j.tics.2003.10.013
Danilov, Y. P., Tyler, M. E., Rust, K. L., Kaczmarek, K. A., & Subbotin, A. M. (N.D.). Non-invasive neuromodulation to improve gait in chronic Multiple Sclerosis: A randomized controlled trial. Unpublished manuscript.
Kaczmarek, K. A. (2011). The tongue display unit for electroctactile spatiotemporal pattern presentation. Scientia Iranica, D18(6), 1476-1485.
Kern, D. S., & Kumar, R. (2007). Deep brain stimulation. [Review]. The Neurologist, 13, 237-252. doi: 10.1097/NRL.0b013e3181492c48
Kratochwill, T. R., Hitchcock, J., Horner, R. H., Levin, J. R., Odom, S. L., Rindskopf, D. M & Shadish, W. R. (2010). Single-case designs technical documentation. Retrieved from What Works Clearinghouse website: http://ies.ed.gov/ncee/wwc/pdf/wwc_scd.pdf.
Nourbakhsh, M. R., & Ottenbacher, K. J. (1994). The statistical analysis of single-subject data: A comparative examination. Physical Therapy, 74, 768-776.
O'Malley, M. K., Ro, T., & Levin, H. S. (2006). Assessing and inducing neuroplasticity with transcranial magnetic stimulation and robotics for motor function. Archives of Physical Medicine & Rehabilitation, 87(12 Suppl 2), S59-S66.
Ottenbacher, K. J. (1986). Reliability and accuracy of visually analyzing graphed data from single-subject designs. American Journal of Occupational Therapy, 40(7), 464-469.
Parker, R. I., Vannest, K. J., & Davis, J. L. (2011). Effect size in single-case research: A review of nine overlap techniques. Behavior Modification, 1-20. doi: 10.1177/0145445511399147
Solanas, A., Manolov, R., & Onghena, P. (2010). Estimating slope and level change in N = 1 designs. Behavior Modification, 34(3), 195-218.
Tactile Communication & Neurorehabilitation Laboratory. (2011). Manual: Balance and gait training for subjects with Multiple Sclerosis. Reducing symptoms of MS using Cranial Nerve Noninvasive Neuromodulation (CN-NINM).
Wildenberg, J. C., Tyler, M. E., Danilov, Y. P., Kaczmarek, K. A., & Meyerand, M. E. (2010). Sustained cortical and subcortical neuromodulation induced by electrical tongue stimulation. Brain Imaging and Behavior, 4, 199-211. doi: 10.1007/s11682-010-9099-7
Wildenberg, J. C., Tyler, M. E., Danilov, Y. P., Kaczmarek, K. A., & Meyerand, M. E. (2011). High-resolution fMRI detects neuromodulation of individual brainstem nuclei by electrical tongue stimulation in balance-impaired individuals. NeuroImage, 56, 2129-2137. doi: 10.1016/j.neuroimage.2011.03.074